ZnS Quantum Dot Based Acetone Sensor for Monitoring Health-Hazardous Gases in Indoor/Outdoor Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Material Characterizations
2.3. ZnS Quantum Dots (QDs) Based Gas Sensor Fabrication and Measurements
3. Results and Discussion
3.1. Structural and Morphological Studies
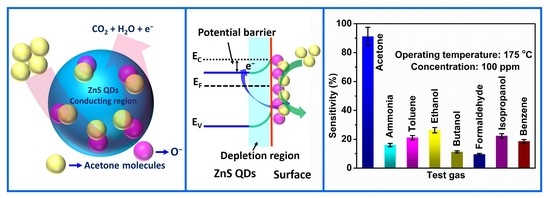
3.2. Acetone Gas Sensing Studies
3.3. Gas Sensing Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, Y.; Ren, B.; Xu, K.; Jeerapan, I.; Chen, H.; Li, Z.; Ou, J.Z. Recent progress in intrinsic and stimulated room-temperature gas sensors enabled by low-dimensional materials. J. Mater. Chem. C 2021, 9, 30263051. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Yang, C.; Liu, X.; Zhang, J. Highly sensitive and selective electronic sensor based on Co catalyzed SnO2 nanospheres for acetone detection. Sens. Actuators B 2020, 304, 127237. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Zhou, M.; Zhang, S.; Cao, S.; Lei, G.; Lou, C.; Zhang, J. Plasma-induced oxygen vacancies enabled ultrathin ZnO films for highly sensitive detection of trimethylamine. J. Hazard. Mater. 2021, 415, 125757. [Google Scholar] [CrossRef]
- Wang, J.; Shen, H.; Xia, Y.; Komarneni, S. Light-activated room-temperature gas sensors based on metal oxide nanostructures: A review on recent advances. Ceram. Int. 2021, 47, 7353–7368. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Oxygen Vacancies Enabled Porous SnO2 Thin Films for Highly Sensitive Detection of Triethylamine at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 20704–20713. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar]
- Wang, C.; Wang, Y.; Cheng, P.; Xu, L.; Dang, F.; Wang, T.; Lei, Z. In-situ generated TiO2/α-Fe2O3 heterojunction arrays for batch manufacturing of conductometric acetone gas sensors. Sens. Actuators B 2021, 340, 129926. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. Ordered mesoporous Ag-doped TiO2/SnO2 nanocomposite based highly sensitive and selective VOC sensors. J. Mater. Chem. A 2016, 4, 1033–1043. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Pokorski, M.; Sartucci, F.; Domenici, L.; Giulio, C.D. Volatile organic compounds (VOCs) fingerprint of Alzheimer’s disease. Respir. Physiol. Neurobiol. 2015, 209, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C. Measuring breath acetone for monitoring fat loss: Review. Obesity 2015, 23, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wang, Z.; Yuan, Y.; Chen, Z.; Zhao, X.; Li, Y.; Wang, C. Continuous monitoring of breath acetone, blood glucose and blood ketone in 20 type 1 diabetic outpatients over 30 days. J. Anal. Bioanal. Tech. 2017, 8, 2155–9872. [Google Scholar]
- Storer, M.; Dummer, J.; Lunt, H.; Scotter, J.; McCartin, F.; Cook, J.; Swanney, M.; Kendall, D.; Logan, F.; Epton, M. Measurement of breath acetone concentrations by selected ion flow tube mass spectrometry in type 2 Diabetes. J. Breath Res. 2011, 5, 046011. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lou, C.; Zheng, L.; Zheng, W.; Liu, X.; Kumar, M.; Zhang, J. Highly sensitive and selective detection of acetone based on platinum sensitized porous tungsten oxide nanospheres. Sens. Actuators B 2020, 307, 127616. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.-J.; Lee, I.; Youn, D.-Y.; Park, C.O.; Lee, J.-H.; Tuller, H.L.; Kim, I.-D. Thin-Wall Assembled SnO2 Fibers Functionalized by Catalytic Pt Nanoparticles and their Superior Exhaled-Breath-Sensing Properties for the Diagnosis of Diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Ebeler, S.E.; Clifford, A.J.; Shibamoto, T. Quantitative analysis by gas chromatography of volatile carbonyl compounds in expired air from mice and human. J. Chromatogr. B Biomed. Sci. Appl. 1997, 702, 211–215. [Google Scholar] [CrossRef]
- Potter, D.W.; Pawliszyn, J. Detection of substituted benzenes in water at the pg/mL level using solid-phase microextraction and gas chromatography-ion trap mass spectrometry. J. Chromatogr. 1992, 625, 247–255. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for Enhanced Detection of Acetone as a Potential Tool for Noninvasive Diabetes Monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Kato, F.; Nakamura, N.; Oshikane, Y.; Nagakubo, A.; Ogi, H. MEMS hydrogen gas sensor with wireless quartz crystal resonator. Sens. Actuators B 2021, 334, 129651. [Google Scholar] [CrossRef]
- Jie, J.S.; Zhang, W.J.; Jiang, Y.; Meng, X.M.; Zapien, J.A.; Shao, M.W.; Lee, S.T. Heterocrystal and bicrystal structures of ZnS nanowires synthesized by plasma enhanced chemical vapour deposition. Nanotechnology 2006, 17, 2913. [Google Scholar] [CrossRef]
- Jiang, P.; Jie, J.; Yu, Y.; Wang, Z.; Xie, C.; Zhang, X.; Wu, C.; Wang, L.; Zhu, Z.; Luo, L. Aluminium-doped n-type ZnS nanowires as high-performance UV and humidity sensors. J. Mater. Chem. 2012, 22, 6856–6861. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Z.; Huang, H.; Liu, Z.; Chen, D.; Shen, G. Gas sensors, thermistor and photodetector based on ZnS nanowires. J. Mater. Chem. 2012, 22, 6845–6850. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, R.; Zhu, Z.; Wang, S. Au nanoparticles decorated ZnS hollow spheres for highly improved gas sensor performances. Sens. Actuators B 2017, 245, 112–121. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, S.Y.; Xu, X.L.; Jiao, H.Y.; Zhang, G.H.; Liu, L.W.; Wang, P.Y.; Gengzang, D.J.; Yao, H.H. Optimization ethanol detection performance manifested by gas sensor based on In2O3/ZnS rough microspheres. Sens. Actuators B 2018, 264, 263–278. [Google Scholar] [CrossRef]
- Gang, Z.; Pei, Z.; Tongjun, N.; Lin, L.; Jiatao, D.; Yong, J.; Zhifeng, J.; Xiaosong, S. Synthesis and characterization of ZnS nanotubes assisted by ethylene glycol. Mater. Lett. 2017, 189, 263–266. [Google Scholar] [CrossRef]
- Verma, N.; Singh, A.K.; Saini, N. Synthesis and characterization of ZnS quantum dots and application for development of arginine biosensor. Sens. Bio-Sens. Res. 2017, 15, 41–45. [Google Scholar] [CrossRef]
- Peng, Q.; Jie, J.; Xie, C.; Wang, L.; Zhang, X.; Wu, D.; Yu, Y.; Wu, C.; Wang, Z.; Jiang, P. Nano-Schottky barrier diodes based on Sb-doped ZnS nanoribbons with controlled p-type conductivity. Appl. Phys. Lett. 2011, 98, 123117. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Wang, Q. Ultrathin single crystal ZnS nanowires. Chem. Commun. 2010, 46, 8941–8943. [Google Scholar] [CrossRef]
- Guijarro, N.; Campiña, J.M.; Shen, Q.; Toyoda, T.; Lana-Villarreal, T.; Gómez, R. Uncovering the role of the ZnS treatment in the performance of quantum dot sensitized solar cells. Phys. Chem. Chem. Phys. 2011, 13, 12024–12032. [Google Scholar] [CrossRef]
- Katayama, H.; Oda, S.; Kukimoto, H. ZnS blue-light-emitting diodes with an external quantum efficiency of 5×10−4. Appl. Phys. Lett. 1975, 27, 697. [Google Scholar] [CrossRef]
- Tauchi, T.; Yamada, Y.; Ohno, T.; Mullins, J.T.; Masumoto, Y. Ultraviolet laser and photodetector of CdZnS/ZnS multiple quantum wells. Phys. B Condens. Matter. 1993, 191, 136–139. [Google Scholar] [CrossRef]
- Su, D.; Kretschmer, K.; Wang, G. Improved Electrochemical Performance of Na-Ion Batteries in Ether-Based Electrolytes: A Case Study of ZnS Nanospheres. Adv. Energy Mater. 2016, 6, 1501785. [Google Scholar] [CrossRef]
- Yi, T.-F.; Li, Y.; Li, Y.-M.; Luo, S.; Liu, Y.-G. ZnS nanoparticles as the electrode materials for high-performance supercapacitors. Solid State Ion. 2019, 343, 115074. [Google Scholar] [CrossRef]
- Tsai, Y.-S.; Chou, T.-W.; Xu, C.Y.; Huang, W.; Lin, C.F.; Wu, Y.S.; Lin, Y.-S.; Chen, H. ZnO/ZnS core-shell nanostructures for hydrogen gas sensing performances. Ceram. Int. 2019, 45, 177551–177557. [Google Scholar] [CrossRef]
- Chang, X.; Qiao, X.; Li, K.; Wang, P.; Xiong, Y.; Li, X.; Xia, F.; Xue, Q. UV assisted ppb-level acetone detection based on hollow ZnO/MoS2 nanosheets core/shell heterostructures at low temperature. Sens. Actuators B 2020, 317, 1282082. [Google Scholar] [CrossRef]
- Jahromi, H.D.; Kazemi, M.; Sheikhi, M.H. Room temperature and highly sensitive acetone sensor based on lead sulfide nanosheets. Mater. Sci. Eng. B 2021, 267, 115082. [Google Scholar] [CrossRef]
- Ma, Y.T.; Ma, S.Y.; Tang, J.; Wu, Z.G.; Shi, J.; Zhao, Y.; Pei, S.T. One-pot hydrothermal method synthesised SnS/rGO nanocomposite under PVDF bonding for high-performance acetone gas sensor. Mater. Sci. Eng. B 2021, 263, 114861. [Google Scholar] [CrossRef]
- Jayababu, N.; Poloju, M.; Shruthi, J.; Reddy, M.V.R. NiO decorated CeO2 nanostructures as room temperature isopropanol gas sensors. RSC Adv. 2019, 9, 13765–13775. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.K.; Shukla, P.K. Effect of sulphur concentration on the structural and electronic properties of ZnS nanoparticles synthesized using chemical precipitation method. J. Mater. Sci. Mater. Electron. 2017, 28, 6226–6232. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley: Reading, MA, USA, 1978. [Google Scholar]
- Su, P.-G.; Lin-Kuo, S. H2-gas sensing and discriminating actions of a single-yarn sensor based on a Pd/GO multilayered thin film using FFT. Anal. Methods 2020, 12, 3537–3544. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, J.; Zhang, Y.; Tian, F.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J.; Pinna, N. Edge-enriched WS2 nanosheets on carbon nanofibers boosts NO2 detection at room temperature. J. Hazard. Mater. 2021, 411, 125120. [Google Scholar] [CrossRef]
- Jung, G.; Shin, W.; Hong, S.; Jeong, Y.; Park, J.; Kim, D.; Bae, J.-H.; Park, B.-G.; Lee, J.-H. Comparison of the characteristics of semiconductor gas sensors with different transducers fabricated on the same substrate. Sens. Actuators B 2021, 335, 129661. [Google Scholar] [CrossRef]
- Siemons, M.; Simon, U. Gas sensing properties of volume-doped CoTiO3 synthesized via polyol method. Sens. Actuators B 2007, 126, 595–603. [Google Scholar] [CrossRef]
- Li, P.; Cao, C.; Shen, Q.; Bai, B.; Jin, H.; Yu, J.; Chen, W.; Song, W. Cr-doped NiO nanoparticles as selective and stable gas sensor for ppb-level detection of benzyl mercaptan. Sens. Actuators B 2021, 339, 129886. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Y.; Zheng, L.; Yang, C.; Pinna, N.; Liu, X.; Zhang, J. MoS2 Van der Waals p–n Junctions Enabling Highly Selective Room-Temperature NO2 Sensor. Adv. Funct. Mater. 2020, 30, 2000435. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, K.; Li, Q.; Zhu, Q.Z.; Wan, Q. Room-temperature high-sensitivity H2S gas sensor based on dendritic ZnO nanostructures with macroscale in appearance. Appl. Phys. 2008, 103, 104305. [Google Scholar] [CrossRef]
- Yang, J.; Han, W.; Ma, J.; Wang, C.; Shimanoe, K.; Zhang, S.; Sun, Y.; Cheng, P.; Wang, Y.; Zhang, H.; et al. Sn doping effect on NiO hollow nanofibers based gas sensors about the humidity dependence for triethylamine detection. Sens. Actuators B 2021, 340, 129971. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, W.; Liu, X.; Zhang, L.; Zheng, L.; Yang, C.; Pinna, N.; Zhang, J. Platinum single atoms on tin oxide ultrathin films for extremely sensitive gas detection. Mater. Horiz. 2020, 7, 1519–1527. [Google Scholar] [CrossRef]
- Vahl, A.; Lupan, O.; Santos-Carballal, D.; Postica, V.; Hansen, S.; Cavers, H.; Wolf, N.; Terasa, M.-I.; Hoppe, M.; Cadi-Essadek, A.; et al. Surface functionalization of ZnO:Ag columnar thin films with AgAu and AgPt bimetallic alloy nanoparticles as an efficient pathway for highly sensitive gas discrimination and early hazard detection in batteries. J. Mater. Chem. A 2020, 8, 16246–16264. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, R.K.; Choi, G.-J.; Choi, H.-J.; Gwag, J.-S. ZnS Quantum Dot Based Acetone Sensor for Monitoring Health-Hazardous Gases in Indoor/Outdoor Environment. Micromachines 2021, 12, 598. https://doi.org/10.3390/mi12060598
Mishra RK, Choi G-J, Choi H-J, Gwag J-S. ZnS Quantum Dot Based Acetone Sensor for Monitoring Health-Hazardous Gases in Indoor/Outdoor Environment. Micromachines. 2021; 12(6):598. https://doi.org/10.3390/mi12060598
Chicago/Turabian StyleMishra, Rajneesh Kumar, Gyu-Jin Choi, Hyeon-Jong Choi, and Jin-Seog Gwag. 2021. "ZnS Quantum Dot Based Acetone Sensor for Monitoring Health-Hazardous Gases in Indoor/Outdoor Environment" Micromachines 12, no. 6: 598. https://doi.org/10.3390/mi12060598
APA StyleMishra, R. K., Choi, G. -J., Choi, H. -J., & Gwag, J. -S. (2021). ZnS Quantum Dot Based Acetone Sensor for Monitoring Health-Hazardous Gases in Indoor/Outdoor Environment. Micromachines, 12(6), 598. https://doi.org/10.3390/mi12060598