Poly (Ethylene Glycol) Methyl Ether Methacrylate-Based Hydrogel and Cerium(IV) Oxide Nanoparticles as Ophthalmic Lens Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Polymerization
2.3. Analysis
3. Results and Discussion
3.1. Physical Properties
3.1.1. Refractive Index and Water Content
3.1.2. Optical Transmittance
3.1.3. Tensile Strength
3.1.4. Tests for Absorbance and Extractables
3.1.5. Antimicrobial Test
3.2. Surface Property
3.2.1. Wettability
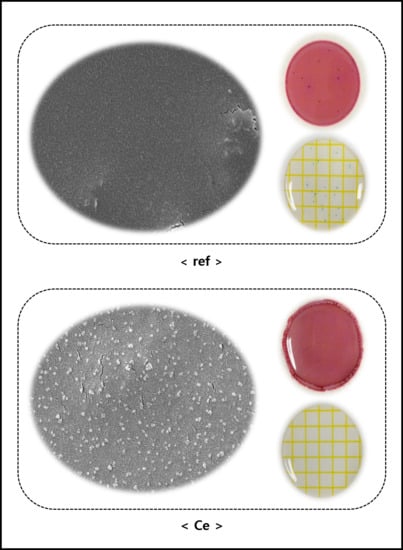
3.2.2. SEM and AFM Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kusuma, V.A.; Gunawan, G.; Smith, Z.P. Gas permeability of cross-linked poly (ethylene-oxide) based on poly (ethylene glycol) dimethacrylate and a miscible siloxane comonomer. Polymer 2010, 51, 5734–5743. [Google Scholar] [CrossRef]
- Parsons, C.; McCoy, C.P.; Gorman, S.P. Anti-infective photodynamic biomaterials for the prevention of intraocular lens-associated infectious endophthalmitis. Biomaterials 2009, 30, 597–602. [Google Scholar] [CrossRef]
- Murakami, K.; Aok, H.; Nakamura, S. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado-Codina, C.; Efron, N. Hydrogel lenses-materials and manufacture: A review. Opt. Prac. 2003, 4, 101–113. [Google Scholar]
- Sariri, R.; Sabbaghzadeh, R. Competitive adsorption of proteins on hydrogel contact lenses. CLAO J. 2001, 27, 159–162. [Google Scholar]
- Tranoudis, I.; Efron, N. Parameter stability of soft contact lenses made from different materials. Contact Lens Anterior Eye 2004, 27, 115–131. [Google Scholar] [CrossRef]
- Masnick, K.B.; Holden, B.A. A study of water content and parametric variations of hydrophilic contact lenses. Aust. J. Optom. 1972, 55, 481–487. [Google Scholar] [CrossRef]
- Lim, S.K.; Lee, S.K.; Hwang, S.H.; Kim, H. Photocatalytic deposition of silver nanoparticles onto organic/inorganic composite nanofibers. Macromol. Mater. Eng. 2006, 291, 1265–1270. [Google Scholar] [CrossRef]
- Li, J.X.; Wang, L.; Shenm, R.L.; Xum, Z.J.; Li, P.; Wan, G.J.; Huang, N. The influence of polyethylene terephthalate surfaces modified by silver ion implantation on bacterial adhesion behavior. Surf. Coat. Technol. 2007, 201, 8155–8159. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Zuclich, J.A. Ultraviolet-induced photochemical damage in ocular tissues. Health Phys. 1989, 56, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.A.; Fitzpatrick, T.B.; Greiter, F.J.; Kraus, E.W. Principles of photoprotection in sunburn and suntanning, and topical and systemic photo-protection in health and diseases. J. Dermatol. Surg. Oncol. 1985, 11, 575–579. [Google Scholar] [CrossRef]
- Taylor, H.R.; West, S.K.; Rosenthal, F.S.; Muñoz, B.; Newland, H.S.; Abbey, H.; Emmett, E.A. Effect of ultraviolet radiation on cataract formation. N. Engl. J. Med. 1988, 319, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Johar, S.R.; Rawal, U.M.; Jain, N.K.; Vasavada, A.R. Sequential effects of ultraviolet radiation on the histomorphology, cell density and antioxidative status of the lens epithelium—An in vivo study. Photochem. Photobiol. 2003, 78, 306–311. [Google Scholar] [CrossRef]
- Wegener, A.R. In vivo studies on the effect of UV-radiation on the eye lens in animals. Doc. Ophthalmol. 1995, 88, 221–232. [Google Scholar] [CrossRef]
- Clark, S.M.; Doughty, M.K.; Cullen, A.P. Acute effects of ultraviolet-B irradiation on the corneal surface of the pigmented rabbit studied by quantitative scanning electron microscopy. Acta Ophthalmolol. 1990, 68, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Bova, L.M.; Sweeney, M.H.; Jamie, J.F.; Truscott, R.J. Major changes in human ocular UV protection with age. Invest. Ophthalmol. Vis. Sci. 2001, 42, 200–205. [Google Scholar] [PubMed]
- Mainster, M.A.; Tumer, P.L. Ultraviolet-B phototoxicity and hypothetical photomelanomagenesis: Intraocular and crystalline lens photoprotection. Am. J. Ophthalol. 2010, 149, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Effiong, P.; Fei, C.C. Metal oxide nanoparticles in biomedical applications. In Metal Oxide Powder Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 233–251. [Google Scholar]
- Hosseini, M.; Mozafari, M. Cerium oxide nanoparticles: Recent advances in tissue engineering. Materials 2020, 13, 3072. [Google Scholar] [CrossRef]
- Choi, S.W.; Cha, B.G.; Kim, J.Y. Therapeutic contact lens for scavenging excessive reactive oxygen species on the ocular surface. ACS Nano 2020, 14, 2483–2496. [Google Scholar] [CrossRef]
- Maccarone, R.; Tisi, A.; Passacantando, M.; Ciancaglini, M. Ophthalmic applications of cerium oxide nanoparticles. J. Ocul. Pharmacol. Ther. 2020, 36, 376–383. [Google Scholar] [CrossRef]
- Shinryo, Y.; Tsugio, S. Cerium oxide for sunscreen cosmetics. J. Solid State Chem. 2003, 171, 7–11. [Google Scholar]
- Zholobak, N.M.; Ivanov, V.K.; Shcherbakov, A.B.; Shaporev, A.S.; Polezhaeva, O.S.; Baranchikov, A.Y.; Spivak, N.Y.; Tretyakov, Y.D. UV-shielding property, photocatalytic activity and photocytotoxicity of ceria colloid solutions. J. Photochem. Photobiol. B 2011, 102, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Truffault, L.; Winton, B.; Choquenet, B.; Andreazza, C.; Simmonard, C.; Devers, T.; Konstantinov, K.; Couteau, C.; Coiffard, L.J.M. Cerium oxide based particles as possible alternative to ZnO in sunscreens: Effect of the synthesis method on the photoprotection results. Mater. Lett. 2012, 68, 357–360. [Google Scholar] [CrossRef]
- Faure, B.; Salazar-Alvarez, G.; Ahniyaz, A.; Villaluenga, I.; Berriozabal, G.; De Miguel, Y.R.; Bergström, L. Dispersion and surface functionalization of oxide nanoparticles for transparent photocatalytic and UV-protecting coatings and sunscreens. Sci. Technol. Adv. Mater. 2013, 14, 023001. [Google Scholar] [CrossRef] [PubMed]
- Mousa, G.Y.; Callender, M.G.; Sivak, J.G.; Edan, D.J. The effects of hydration characteristics of hydrogel lenses on the refractive index. Int. Contact. Lens. Clin. 1983, 10, 31–37. [Google Scholar]
- Elisseeff, J.; Puleo, C.; Yang, F.; Sharma, B. Advances in skeletaltissue engineering with hydrogels. Orthod. Craniofac. Res. 2005, 8, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Andrew, T.M. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Lee, M.J.; Sung, A.Y. Polymerization and preparation of functional ophthalmic material containing carbon nanoparticles. Korean J. Mater. Res. 2018, 28, 452–458. [Google Scholar] [CrossRef]
- Sun, F.; Cao, P.; Xu, J. Enhancing hydrophilicity and protein resistance of silicone hydrogels by plasma induced grafting with hydrophilic polymers. Chin. J. Polym. Sci. 2010, 28, 547–554. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Reukov, V.V.; Yakimansky, A.V.; Krasnopeeva, E.L.; Ivanova, O.S.; Popov, A.L.; Ivanov, V.K. CeO2 nanoparticle-containing polymers for biomedical applications: A review. Polymers 2021, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.B.; Shin, K.S.; Hwang, T.S. Synthesis and PSA properties of acryl modified resin for semiconductor Wafer. J. Adhes. Interface 2010, 11, 63–69. [Google Scholar]
- Jayashree, B.S. A descriptive study of the regulations of leachable and extractables of US, Europe, and Canada. Int. J. Pharm. Pharmacol. 2018, 2, 1–15. [Google Scholar]
- Biological Evaluation of Medical Devices—Part 18: Chemical Characterization of Materials; ISO 10993-18; International Organization for Standardization (ISO): Geneva, Switzerland, 2020.
- Zholobak, N.; Ivanov, V.; Shcherbakov, A. Interaction of nanoceria with microorganisms. Nanobiomater. Antimicrob. Ther. 2016, 12, 419–450. [Google Scholar]
- Lee, M.J.; Sung, A.Y. Polymerization and preparation of high functional ophthalmic lens material containing 2-fluoro styrene with si and ag nanoparticles. Sci. Adv. Mater. 2020, 12, 427–434. [Google Scholar] [CrossRef]
- Lee, K.J.; Mun, M.Y. Changes in objective and subjective responses in soft contact lens wearers refitted to daily-wear silicone hydrogel contact lenses. J. Korean Oph. Opt. Soc. 2007, 12, 43–54. [Google Scholar]
- Cho, S.A.; Kim, T.H.; Sung, A.Y. Polymerization and characterization of ophthalmic polymer containing glycerol dimethacrylate with High Wettability. J. Korean Chem. 2011, 55, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Kim, T.H.; Sung, A.Y. Study on the strength and surface characteristics of ophthalmic copolymer with glycol group. J. Korean Chem. Soc. 2012, 56, 297–302. [Google Scholar] [CrossRef]
- Ye, K.H.; Kim, T.H.; Sung, A.Y. Study on the water content variation of contact lens with silicone type. Korean J. Vis. Sci. 2008, 10, 63–70. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]










| HEMA | Ce * | PEGMA | TEPI | EGDMA | AIBN | Total | |
|---|---|---|---|---|---|---|---|
| ref | 98.72 | - | - | - | 0.99 | 0.2 | 100 |
| Ce | 98.72 | 0.1 | - | - | 0.99 | 0.2 | 100 |
| CeP1 | 97.74 | 0.1 | 0.98 | - | 0.99 | 0.2 | 100 |
| CeP3 | 95.84 | 0.1 | 2.88 | - | 0.99 | 0.2 | 100 |
| CeP5 | 94.01 | 0.1 | 4.71 | - | 0.99 | 0.2 | 100 |
| CeP10 | 89.74 | 0.1 | 8.98 | - | 0.99 | 0.2 | 100 |
| CeP10_ISO1 | 88.85 | 0.1 | 8.89 | 0.98 | 0.99 | 0.2 | 100 |
| CeP10_ ISO 3 | 87.13 | 0.09 | 8.72 | 2.88 | 0.99 | 0.2 | 100 |
| CeP10_ ISO 5 | 85.47 | 0.09 | 8.56 | 4.71 | 0.99 | 0.2 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-J.; Park, S.-Y.; Sung, A.-Y. Poly (Ethylene Glycol) Methyl Ether Methacrylate-Based Hydrogel and Cerium(IV) Oxide Nanoparticles as Ophthalmic Lens Material. Micromachines 2021, 12, 1111. https://doi.org/10.3390/mi12091111
Lee M-J, Park S-Y, Sung A-Y. Poly (Ethylene Glycol) Methyl Ether Methacrylate-Based Hydrogel and Cerium(IV) Oxide Nanoparticles as Ophthalmic Lens Material. Micromachines. 2021; 12(9):1111. https://doi.org/10.3390/mi12091111
Chicago/Turabian StyleLee, Min-Jae, Seon-Young Park, and A-Young Sung. 2021. "Poly (Ethylene Glycol) Methyl Ether Methacrylate-Based Hydrogel and Cerium(IV) Oxide Nanoparticles as Ophthalmic Lens Material" Micromachines 12, no. 9: 1111. https://doi.org/10.3390/mi12091111
APA StyleLee, M. -J., Park, S. -Y., & Sung, A. -Y. (2021). Poly (Ethylene Glycol) Methyl Ether Methacrylate-Based Hydrogel and Cerium(IV) Oxide Nanoparticles as Ophthalmic Lens Material. Micromachines, 12(9), 1111. https://doi.org/10.3390/mi12091111