Investigation of Surface Pre-Treatment Methods for Wafer-Level Cu-Cu Thermo-Compression Bonding
Abstract
:1. Introduction
2. Experimental Design and Bonding Process
2.1. Sample Wafer Fabrication
2.2. Pre-Treatment and Bonding Process
3. Bonding Results and Discussion
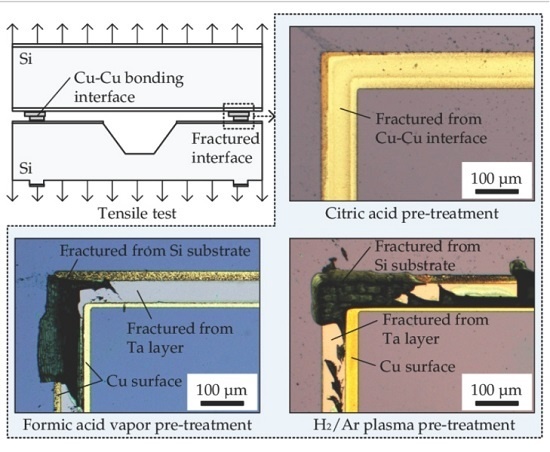
3.1. Tensile Test
3.2. Thermal Desorption Spectroscopy (TDS) Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ashby, M. A first report on deformation-mechanism maps. Acta Metall. 1972, 20, 887–897. [Google Scholar] [CrossRef]
- Derby, B. Solid State Diffusion Bonding: A joining and materials fabrication process. DVS Ber. 1998, 192, 100–103. [Google Scholar]
- Takahashi, Y.; Takahashi, K.; Nishiguchi, K. A numerical analysis of void shrinkage processes controlled by coupled surface and interface diffusion. Acta Metall. Mater. 1991, 39, 3199–3216. [Google Scholar] [CrossRef]
- Froemel, J.; Baum, M.; Wiemer, M.; Roscher, F.; Haubold, M.; Jia, C.; Gessner, T. Investigations of thermocompression bonding with thin metal layers. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; IEEE: New York, NY, USA, 2011; pp. 990–993. [Google Scholar]
- Chen, K.N.; Tan, C.S.; Fan, A.; Reif, R. Morphology and bond strength of copper wafer bonding. Electrochem. Solid-State Lett. 2004, 7, G14–G16. [Google Scholar] [CrossRef]
- Chen, K.N.; Chang, S.M.; Shen, L.C.; Reif, R. Investigations of strength of copper-bonded wafers with several quantitative and qualitative tests. J. Electron. Mater. 2006, 35, 1082–1086. [Google Scholar] [CrossRef]
- Chen, K.N.; Fan, A.; Tan, C.S.; Reif, R. Bonding parameters of blanket copper wafer bonding. J. Electron. Mater. 2006, 35, 230–234. [Google Scholar] [CrossRef]
- Baum, M.; Hofmann, L.; Wiemer, M.; Schulz, S.; Gessner, T. Development and characterisation of 3D integration technologies for MEMS based on copper filled TSV’s and copper-to-copper metal thermo compression bonding. In Proceedings of the 2013 International Semiconductor Conference Dresden-Grenoble (ISCDG), Dresden, Germany, 26–27 September 2013; IEEE: New York, NY, USA, 2013; pp. 1–4. [Google Scholar]
- Rebhan, B.; Hesser, G.; Duchoslav, J.; Dragoi, V.; Wimplinger, M.; Hingerl, K. Low-temperature Cu-Cu wafer bonding. ECS Trans. 2013, 50, 139–149. [Google Scholar] [CrossRef]
- Shigetou, A.; Itoh, T.; Suga, T. Direct bonding of CMP-Cu films by surface activated bonding (SAB) method. J. Mater. Sci. 2005, 40, 3149–3154. [Google Scholar] [CrossRef]
- Yang, W.; Shintani, H.; Akaike, M.; Suga, T. Low temperature Cu-Cu direct bonding using formic acid vapor pretreatment. In Proceedings of the 2011 IEEE 61st Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 31 May–3 June 2011; IEEE: New York, NY, USA, 2011; pp. 2079–2083. [Google Scholar]
- Lim, D.F.; Singh, S.G.; Ang, X.F.; Wei, J.; Ng, C.M.; Tan, C.S. Achieving low temperature Cu to Cu diffusion bonding with self assembly monolayer (SAM) passivation. In Proceedings of the 2009 IEEE International Conference on 3D System Integration, San Francisco, CA, USA, 28–30 September 2009; IEEE: New York, NY, USA, 2009; pp. 1–5. [Google Scholar]
- Tan, C.S.; Lim, D.F.; Singh, S.G.; Goulet, S.K.; Bergkvist, M. Cu-Cu diffusion bonding enhancement at low temperature by surface passivation using self-assembled monolayer of alkane-thiol. Appl. Phys. Lett. 2009, 95, 192108. [Google Scholar] [CrossRef]
- Baklanov, M.R.; Shamiryan, D.G.; Tokei, Z.; Beyer, G.P.; Conard, T.; Vanhaelemeersch, S.; Maex, K. Characterization of Cu surface cleaning by hydrogen plasma. J. Vac. Sci. Technol. B Microelectron. Nanomater. Struct. 2001, 19, 1201–1211. [Google Scholar] [CrossRef]
- Chang, Y.; Leu, J.; Lin, B.; Wang, Y.; Cheng, Y. Comparison of H2 and NH3 Treatments for Copper Interconnects. Adv. Mater. Sci. Eng. 2013, 2013, 825195. [Google Scholar] [CrossRef]
- Ohashi, O.; Hashimoto, T. Study on Diffusion Welding (Report 2). J. Jpn. Weld. Soc. 1976, 45, 295–301. [Google Scholar] [CrossRef]
- Nosaka, T.; Yoshitake, M.; Okamoto, A.; Ogawa, S.; Nakayama, Y. Thermal decomposition of copper nitride thin films and dots formation by electron beam writing. Appl. Surf. Sci. 2001, 169–170, 358–361. [Google Scholar] [CrossRef]
- Maestre Caro, A.; Travaly, Y.; Beyer, G.; Tokei, Z.; Maes, G.; Borghs, G.; Armini, S. Selective self-assembled monolayer coating to enable Cu-to-Cu connection in dual damascene vias. Microelectron. Eng. 2013, 106, 76–80. [Google Scholar] [CrossRef]
- Hirashita, N.; Uchiyama, T. Quantitative desorption analysis of ultra large scale integration materials by thermal desorption spectroscopy. Bunseki Kagaku 1994, 43, 757–764. [Google Scholar] [CrossRef]
- Hirashita, N.; Urano, M.; Yoshida, H. Measurements of Outgassing Rates in a Field of Analysis. J. Vac. Soc. Jpn. 2014, 57, 214–218. [Google Scholar] [CrossRef]
- Vidali, G.; Ihm, G.; Kim, H.-Y.; Cole, M.W. Potentials of physical adsorption. Surf. Sci. Rep. 1991, 12, 135–181. [Google Scholar] [CrossRef]
- Christmann, K. Interaction of hydrogen with solid surfaces. Surf. Sci. Rep. 1988, 9, 1–163. [Google Scholar] [CrossRef]
- Nobuhara, K.; Nakanishi, H.; Kasai, H.; Okiji, A. Behavior of H atom in adsorption states on metal surfaces–localization and delocalization. Surf. Sci. 2001, 493, 271–277. [Google Scholar] [CrossRef]
- Jewell, A.D.; Peng, G.; Mattera, M.F.G.; Lewis, E.A.; Murphy, C.J.; Kyriakou, G.; Mavrikakis, M.; Sykes, E.C.H. Quantum Tunneling Enabled Self-Assembly of Hydrogen Atoms on Cu(111). ACS Nano 2012, 6, 10115–10121. [Google Scholar] [CrossRef] [PubMed]







| Pre-Treatment | Code | Temperature | Time | Remarks |
|---|---|---|---|---|
| Formic acid vapor | FV-3x | 300 °C | 3 × 10 min | Three-step heating up to 300 °C, each heating step takes 10 min. Pressure of formic acid vapor was 100 kPa. Performed in the bonding chamber and bonded without Cu oxide layer to compare others. |
| H2/Ar plasma | PH-350 | 350 °C | 30 s | H2/Ar flow rate, RF power and pressure were 300/140 sccm, 100 W and 130 Pa, respectively. Oxide can be removed by H radical and Ar ion. H atom chemisorption on Cu surface is expected. |
| NH3 plasma | PN-350 | 350 °C | 60 s | NH3 flow rate, RF power and pressure were 220 sccm, 100 W and 130 Pa, respectively. Oxide can be removed by radicals. Cu3N is expected to be formed on Cu surface. |
| Hexanethiol SAM | SH-200 | Room temperature | 2 h | 1-Hexanethiol (95%) was dissolved in 2-propanol at 1 mM of concentration. Native oxide was removed by citric acid. SAM film was tried to be desorbed by heating at 200 °C for 10 min before bonding. |
| Decanethiol SAM | SD-200 | Room temperature | 2 h | 1-Decanethiol (95%) was dissolved in 2-propanol at 1 mM of concentration. Native oxide was removed by citric acid. SAM film was tried to be desorbed by heating at 200 °C for 10 min before bonding. |
| Citric acid solution | CA | Room temperature | 1 min | 1 wt % citric acid solution in DI water. A pair of the wafers was immersed into the citric acid solution, and bonded with re-oxidized Cu oxide layer to compare with others. |
| Code | Temperature | Force | Duration |
|---|---|---|---|
| B-300 | 300 °C | 30 kN | 60 min |
| B-250 | 250 °C | 30 kN | 60 min |
| B-200 | 200 °C | 30 kN | 60 min |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, K.; Wang, W.-S.; Baum, M.; Froemel, J.; Hirano, H.; Tanaka, S.; Wiemer, M.; Otto, T. Investigation of Surface Pre-Treatment Methods for Wafer-Level Cu-Cu Thermo-Compression Bonding. Micromachines 2016, 7, 234. https://doi.org/10.3390/mi7120234
Tanaka K, Wang W-S, Baum M, Froemel J, Hirano H, Tanaka S, Wiemer M, Otto T. Investigation of Surface Pre-Treatment Methods for Wafer-Level Cu-Cu Thermo-Compression Bonding. Micromachines. 2016; 7(12):234. https://doi.org/10.3390/mi7120234
Chicago/Turabian StyleTanaka, Koki, Wei-Shan Wang, Mario Baum, Joerg Froemel, Hideki Hirano, Shuji Tanaka, Maik Wiemer, and Thomas Otto. 2016. "Investigation of Surface Pre-Treatment Methods for Wafer-Level Cu-Cu Thermo-Compression Bonding" Micromachines 7, no. 12: 234. https://doi.org/10.3390/mi7120234
APA StyleTanaka, K., Wang, W. -S., Baum, M., Froemel, J., Hirano, H., Tanaka, S., Wiemer, M., & Otto, T. (2016). Investigation of Surface Pre-Treatment Methods for Wafer-Level Cu-Cu Thermo-Compression Bonding. Micromachines, 7(12), 234. https://doi.org/10.3390/mi7120234