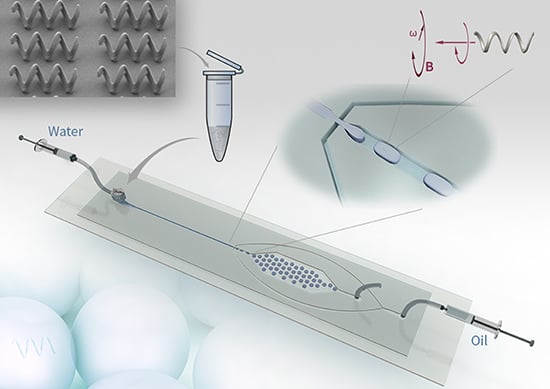
Microfluidic-Based Droplet and Cell Manipulations Using Artificial Bacterial Flagella
Abstract
:1. Introduction
2. Experiment, Results and Discussion
2.1. Operation inside Microfluidic Droplet


2.2. Manipulation of Microdroplets



2.3. Motorized Cell

3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
1. ABF Fabrication and Suspension Preparation
2. PDMS Chip Fabrication and ABF Encapsulation
3. Magnetic Control System
4. Method to Assess Swimming Steadiness

5. Treatment of Biological Cells
6. Cell Viability Assessment
| Time | Living Cell Proportion (Control) | Living Cell Proportion (Test) |
|---|---|---|
| 15 min | 97.3% | 98.6% |
| 30 min | 96.2% | 97.6% |
| 60 min | 96.1% | 96.3% |
| 120 min | 94.0% | 94.5% |

References
- Nelson, B.J.; Kaliakatsos, I.K.; Abbott, J.J. Microrobots for Minimally Invasive Medicine. Annu. Rev. Biomed. Eng. 2010, 12, 55–85. [Google Scholar] [CrossRef] [PubMed]
- Peyer, K.E.; Zhang, L.; Nelson, B.J. Bio-inspired magnetic swimming microrobots for biomedical applications. Nanoscale 2013, 5, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.M.; Trichet-Paredes, C.; Sánchez, S. Applications of three-dimensional (3D) printing for microswimmers and bio-hybrid robotics. Lab Chip 2015, 15, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Servant, A.; Qiu, F.; Mazza, M.; Kostarelos, K.; Nelson, B.J. Controlled In Vivo Swimming of a Swarm of Bacteria-Like Microrobotic Flagella. Adv. Mater. 2015, 27, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.C.; Anderson, R.A. Bacteria Swim by Rotating their Flagellar Filaments. Nature 1973, 245, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, R.; Baudry, J.; Roper, M.L.; Fermigier, M.; Stone, H.A.; Bibette, J. Microscopic artificial swimmers. Nature 2005, 437, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peyer, K.E.; Petit, T.; Kratochvil, B.E.; Nelson, B.J. Motion control of artificial bacterial flagella. In Proceedings of 2010 10th IEEE Conference on Nanotechnology (IEEE-NANO), Seoul, Korea, 17–20 August 2010; pp. 893–896.
- Gao, W.; Manesh, K.M.; Hua, J.; Sattayasamitsathit, S.; Wang, J. Hybrid Nanomotor: A Catalytically/Magnetically Powered Adaptive Nanowire Swimmer. Small 2011, 7, 2047–2051. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.G.; Fischer, P. Active colloidal microdrills. Chem. Commun. 2015, 51, 4192–4195. [Google Scholar] [CrossRef] [PubMed]
- Den Toonder, J.; Bos, F.; Broer, D.; Filippini, L.; Gillies, M.; de Goede, J.; Mol, T.; Reijme, M.; Talen, W.; Wilderbeek, H.; Khatavkar, V.; Anderson, P. Artificial cilia for active micro-fluidic mixing. Lab Chip 2008, 8, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Casadevall i Solvas, X.; Lambert, R.A.; Kulinsky, L.; Rangel, R.H.; Madou, M.J. Micromixing and flow manipulation with polymer microactuators. Microfluid. Nanofluid. 2011, 11, 405–416. [Google Scholar] [CrossRef]
- Zhang, L.; Abbott, J.J.; Dong, L.; Kratochvil, B.E.; Zhang, H.; Peyer, K.E.; Nelson, B.J. Micromanipulation using artificial bacterial flagella. In Proceedings of 2009 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2009), St. Louis, MO, USA, 10–15 October 2009; pp. 1401–1406.
- Zhang, L.; Abbott, J.J.; Dong, L.; Peyer, K.E.; Kratochvil, B.E.; Zhang, H.; Bergeles, C.; Nelson, B.J. Characterizing the Swimming Properties of Artificial Bacterial Flagella. Nano Lett. 2009, 9, 3663–3667. [Google Scholar] [CrossRef] [PubMed]
- Peyer, K.E.; Qiu, F.; Zhang, L.; Nelson, B.J. Movement of artificial bacterial flagella in heterogeneous viscous environments at the microscale. In Proceedings of 2012 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2012), Vilamoura, Portugal, 7–12 October 2012; pp. 2553–2558.
- Temel, F.Z.; Erman, A.G.; Yesilyurt, S. Characterization and Modeling of Biomimetic Untethered Robots Swimming in Viscous Fluids Inside Circular Channels. IEEE/ASME Trans. Mechatron. 2014, 19, 1562–1573. [Google Scholar]
- Tottori, S.; Zhang, L.; Qiu, F.; Krawczyk, K.K.; Franco-Obregón, A.; Nelson, B.J. Magnetic Helical Micromachines: Fabrication, Controlled Swimming, and Cargo Transport. Adv. Mater. 2012, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-Y.; Qiu, F.; Tung, H.-W.; Peyer, K.E.; Shamsudhin, N.; Pokki, J.; Zhang, L.; Chen, X.-B.; Nelson, B.J.; Sakar, M.S. Cooperative manipulation and transport of microobjects using multiple helical microcarriers. RSC Adv. 2014, 4, 26771. [Google Scholar] [CrossRef]
- Peters, C.; Ergeneman, O.; García, P.D.W.; Müller, M.; Pané, S.; Nelson, B.J.; Hierold, C. Superparamagnetic Twist-Type Actuators with Shape-Independent Magnetic Properties and Surface Functionalization for Advanced Biomedical Applications. Adv. Funct. Mater. 2014, 24, 5269–5276. [Google Scholar] [CrossRef]
- Qiu, F.; Zhang, L.; Peyer, K.E.; Casarosa, M.; Franco-Obregón, A.; Choi, H.; Nelson, B.J. Noncytotoxic artificial bacterial flagella fabricated from biocompatible ORMOCOMP and iron coating. J. Mater. Chem. B 2013, 2, 357–362. [Google Scholar] [CrossRef]
- Suter, M.; Zhang, L.; Siringil, E.C.; Peters, C.; Luehmann, T.; Ergeneman, O.; Peyer, K.E.; Nelson, B.J.; Hierold, C. Superparamagnetic microrobots: fabrication by two-photon polymerization and biocompatibility. Biomed. Microdevices 2013, 15, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Fujita, S.; Mhanna, R.; Zhang, L.; Simona, B.R.; Nelson, B.J. Magnetic Helical Microswimmers Functionalized with Lipoplexes for Targeted Gene Delivery. Adv. Funct. Mater. 2015, 25, 1666–1671. [Google Scholar] [CrossRef]
- Qiu, F.; Mhanna, R.; Zhang, L.; Ding, Y.; Fujita, S.; Nelson, B.J. Artificial bacterial flagella functionalized with temperature-sensitive liposomes for controlled release. Sens. Actuators B Chem. 2014, 196, 676–681. [Google Scholar] [CrossRef]
- Mhanna, R.; Qiu, F.; Zhang, L.; Ding, Y.; Sugihara, K.; Zenobi-Wong, M.; Nelson, B.J. Artificial Bacterial Flagella for Remote-Controlled Targeted Single-Cell Drug Delivery. Small 2014, 10, 1953–1957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Abbott, J.J.; Dong, L.; Kratochvil, B.E.; Bell, D.; Nelson, B.J. Artificial bacterial flagella: Fabrication and magnetic control. Appl. Phys. Lett. 2009, 94, 064107. [Google Scholar] [CrossRef]
- Cumpston, B.H.; Ananthavel, S.P.; Barlow, S.; Dyer, D.L.; Ehrlich, J.E.; Erskine, L.L.; Heikal, A.A.; Kuebler, S.M.; Lee, I.-Y.S.; McCord-Maughon, D.; et al. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999, 398, 51–54. [Google Scholar]
- Wu, S.; Serbin, J.; Gu, M. Two-photon polymerisation for three-dimensional micro-fabrication. J. Photochem. Photobiol. Chem. 2006, 181, 1–11. [Google Scholar] [CrossRef]
- Von Freymann, G.; Ledermann, A.; Thiel, M.; Staude, I.; Essig, S.; Busch, K.; Wegener, M. Three-Dimensional Nanostructures for Photonics. Adv. Funct. Mater. 2010, 20, 1038–1052. [Google Scholar] [CrossRef]
- Juodkazis, S.; Mizeikis, V.; Seet, K.K.; Miwa, M.; Misawa, H. Two-photon lithography of nanorods in SU-8 photoresist. Nanotechnology 2005, 16, 846. [Google Scholar] [CrossRef]
- De Marco, C.; Gaidukeviciute, A.; Kiyan, R.; Eaton, S.M.; Levi, M.; Osellame, R.; Chichkov, B.N.; Turri, S. A New Perfluoropolyether-Based Hydrophobic and Chemically Resistant Photoresist Structured by Two-Photon Polymerization. Langmuir 2013, 29, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.-F.; Zheng, M.-L.; Duan, X.-M. Two-photon polymerization microfabrication of hydrogels: An advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015, 44, 5031–5039. [Google Scholar] [CrossRef] [PubMed]
- Kintses, B.; van Vliet, L.D.; Devenish, S.R.; Hollfelder, F. Microfluidic droplets: new integrated workflows for biological experiments. Curr. Opin. Chem. Biol. 2010, 14, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Theberge, A.B.; Courtois, F.; Schaerli, Y.; Fischlechner, M.; Abell, C.; Hollfelder, F.; Huck, W.T.S. Microdroplets in Microfluidics: An Evolving Platform for Discoveries in Chemistry and Biology. Angew. Chem. Int. Ed. 2010, 49, 5846–5868. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.G.; Lee, K.-H.; Khademhosseini, A.; Lee, S.-H. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab Chip 2011, 12, 45–59. [Google Scholar] [CrossRef] [PubMed]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Hatch, A.C.; Fisher, J.S.; Tovar, A.R.; Hsieh, A.T.; Lin, R.; Pentoney, S.L.; Yang, D.L.; Lee, A.P. 1-Million droplet array with wide-field fluorescence imaging for digital PCR. Lab Chip 2011, 11, 3838–3845. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.; Demeester, J.; Smedt, S.D. Advanced nanogel engineering for drug delivery. Soft Matter 2009, 5, 707–715. [Google Scholar] [CrossRef]
- Braschler, T.; Johann, R.; Heule, M.; Metref, L.; Renaud, P. Gentle cell trapping and release on a microfluidic chip by in situ alginate hydrogel formation. Lab Chip 2005, 5, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.-H.; Takeuchi, S. Dynamic microarray system with gentle retrieval mechanism for cell-encapsulating hydrogel beads. Lab Chip 2008, 8, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.M.; Alivisatos, A.P.; Mathies, R.A. High-Temperature Microfluidic Synthesis of CdSe Nanocrystals in Nanoliter Droplets. J. Am. Chem. Soc. 2005, 127, 13854–13861. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Ruditskiy, A.; Vara, M.; Xia, Y. Toward continuous and scalable production of colloidal nanocrystals by switching from batch to droplet reactors. Chem. Soc. Rev. 2015, 44, 5806–5820. [Google Scholar] [CrossRef] [PubMed]
- Villar, G.; Graham, A.D.; Bayley, H. A Tissue-Like Printed Material. Science 2013, 340, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Wauer, T.; Gerlach, H.; Mantri, S.; Hill, J.; Bayley, H.; Sapra, K.T. Construction and Manipulation of Functional Three-Dimensional Droplet Networks. ACS Nano 2014, 8, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Brouzes, E.; Medkova, M.; Savenelli, N.; Marran, D.; Twardowski, M.; Hutchison, J.B.; Rothberg, J.M.; Link, D.R.; Perrimon, N.; Samuels, M.L. Droplet microfluidic technology for single-cell high-throughput screening. Proc. Natl. Acad. Sci. USA 2009, 106, 14195–14200. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.E.; Wootton, R.C.R.; deMello, A.J. Continuous and Segmented Flow Microfluidics: Applications in High- throughput Chemistry and Biology. Chim. Int. J. Chem. 2012, 66, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-K.; Monsur Ali, M.; Zhang, K.; Pone, E.J.; Zhao, W. Droplet microfluidics for single-molecule and single-cell analysis in cancer research, diagnosis and therapy. TrAC Trends Anal. Chem. 2014, 58, 145–153. [Google Scholar] [CrossRef]
- Mazutis, L.; Gilbert, J.; Ung, W.L.; Weitz, D.A.; Griffiths, A.D.; Heyman, J.A. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. [Google Scholar] [CrossRef] [PubMed]
- Brouzes, E.; Kruse, T.; Kimmerling, R.; Strey, H.H. Rapid and continuous magnetic separation in droplet microfluidic devices. Lab Chip 2015, 15, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Lauga, E. Life around the scallop theorem. Soft Matter 2011, 7, 3060–3065. [Google Scholar] [CrossRef]
- Li, J. Behaviour of titanium and titania-based ceramics in vitro and in vivo. Biomaterials 1993, 14, 229–232. [Google Scholar] [CrossRef]
- Van Swaay, D.; Mächler, J.-P.; Stanley, C.; deMello, A. A chip-to-world connector with a built-in reservoir for simple small-volume sample injection. Lab Chip 2013, 14, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Kemna, E.W.M.; Schoeman, R.M.; Wolbers, F.; Vermes, I.; Weitz, D.A.; van den Berg, A. High-yield cell ordering and deterministic cell-in-droplet encapsulation using Dean flow in a curved microchannel. Lab Chip 2012, 12, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.R.; Chen, C.-H.; Agresti, J.J.; Weitz, D.A. Beating Poisson encapsulation statistics using close-packed ordering. Lab Chip 2009, 9, 2628–2631. [Google Scholar] [CrossRef] [PubMed]
- Edd, J.F.; Carlo, D.D.; Humphry, K.J.; Köster, S.; Irimia, D.; Weitz, D.A.; Toner, M. Controlled encapsulation of single-cells into monodisperse picolitre drops. Lab Chip 2008, 8, 1262–1264. [Google Scholar] [CrossRef] [PubMed]
- Tottori, S.; Zhang, L.; Peyer, K.E.; Nelson, B.J. Assembly, Disassembly, and Anomalous Propulsion of Microscopic Helices. Nano Lett. 2013, 13, 4263–4268. [Google Scholar] [CrossRef] [PubMed]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction—Scaling and mechanism of break-up. Lab Chip 2006, 6, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Baroud, C.N.; Gallaire, F.; Dangla, R. Dynamics of microfluidic droplets. Lab Chip 2010, 10, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Baret, J.-C. Surfactants in droplet-based microfluidics. Lab Chip 2012, 12, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.J.; Payne, R.J.; Chow, W.Y.; Abell, C. Fluorosurfactants for microdroplets: Interfacial tension analysis. J. Colloid Interface Sci. 2010, 350, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Yu, Y.; Zhu, G.; Jiang, L.; Qin, J. A droplet microchip with substance exchange capability for the developmental study of C. elegans. Lab Chip 2015, 15, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Velev, O.D.; Prevo, B.G.; Bhatt, K.H. On-chip manipulation of free droplets. Nature 2003, 426, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-M.; Kang, I.S. A novel actuation method of transporting droplets by using electrical charging of droplet in a dielectric fluid. Biomicrofluidics 2009, 3, 022402. [Google Scholar] [CrossRef] [PubMed]
- Wixforth, A.; Strobl, C.; Gauer, C.; Toegl, A.; Scriba, J.; Guttenberg, Z.V. Acoustic manipulation of small droplets. Anal. Bioanal. Chem. 2004, 379, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.N.; Valley, J.K.; Neale, S.L.; Jamshidi, A.; Hsu, H.-Y.; Wu, M.C. Light-actuated digital microfluidics for large-scale, parallel manipulation of arbitrarily sized droplets. In Proceedings of 2010 IEEE 23rd International Conference on Micro Electro Mechanical Systems (MEMS 2010), Hong Kong, China, 24–28 January 2010; pp. 252–255.
- Cho, S.K.; Moon, H.; Kim, C.-J. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003, 12, 70–80. [Google Scholar]
- Jebrail, M.J.; Bartsch, M.S.; Patel, K.D. Digital microfluidics: a versatile tool for applications in chemistry, biology and medicine. Lab Chip 2012, 12, 2452–2463. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.M.; Leavitt, P.K.; Thiel, P.A. Oxygenated fluorocarbons adsorbed at metal surfaces: chemisorption bond strengths and decomposition. J. Am. Chem. Soc. 1987, 109, 5621–5627. [Google Scholar] [CrossRef]
- Sakar, M.S.; Steager, E.B.; Kim, D.H.; Kim, M.J.; Pappas, G.J.; Kumar, V. Single cell manipulation using ferromagnetic composite microtransporters. Appl. Phys. Lett. 2010, 96, 043705. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Qiu, F.; Casadevall i Solvas, X.; Chiu, F.W.Y.; Nelson, B.J.; DeMello, A. Microfluidic-Based Droplet and Cell Manipulations Using Artificial Bacterial Flagella. Micromachines 2016, 7, 25. https://doi.org/10.3390/mi7020025
Ding Y, Qiu F, Casadevall i Solvas X, Chiu FWY, Nelson BJ, DeMello A. Microfluidic-Based Droplet and Cell Manipulations Using Artificial Bacterial Flagella. Micromachines. 2016; 7(2):25. https://doi.org/10.3390/mi7020025
Chicago/Turabian StyleDing, Yun, Famin Qiu, Xavier Casadevall i Solvas, Flora Wing Yin Chiu, Bradley J. Nelson, and Andrew DeMello. 2016. "Microfluidic-Based Droplet and Cell Manipulations Using Artificial Bacterial Flagella" Micromachines 7, no. 2: 25. https://doi.org/10.3390/mi7020025
APA StyleDing, Y., Qiu, F., Casadevall i Solvas, X., Chiu, F. W. Y., Nelson, B. J., & DeMello, A. (2016). Microfluidic-Based Droplet and Cell Manipulations Using Artificial Bacterial Flagella. Micromachines, 7(2), 25. https://doi.org/10.3390/mi7020025