Magnetophoretic Sorting of Single Cell-Containing Microdroplets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cultivation of Microalgae
2.3. Device Fabrication
3. Results and Discussion
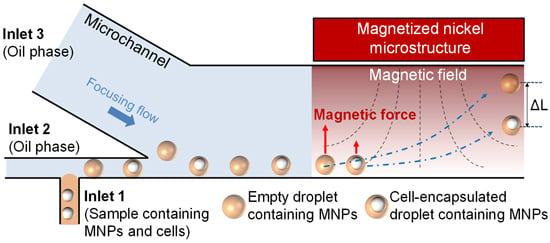
3.1. Design Theory for Magnetophoretic Separation of Single Cell-Containing Microdroplet
3.2. Effect of MNP Concentrations inside the Droplets
3.3. Effect of Magnetophoretic Mobility by the Occupation of a Microbead inside the Droplets
3.4. Magnetophoretic Separation of Single Cell-Encapsulated Droplets
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seeger, S.; Monajembashi, S.; Hutter, K.J.; Futterman, G.; Wolfrum, J.; Greulich, K.O. Application of laser optical tweezers in immunology and molecular-genetics. Cytometry 1991, 12, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Beaujean, F. Methods of CD34+ cell separation: Comparative analysis. Transfus. Sci. 1997, 18, 251–261. [Google Scholar] [CrossRef]
- Walling, M.A.; Shepard, J.R.E. Cellular heterogeneity and live cell arrays. Chem. Soc. Rev. 2011, 40, 4049–4076. [Google Scholar] [CrossRef] [PubMed]
- Davey, H.M.; Kell, D.B. Flow cytometry and cell sorting of heterogeneous microbial populations: The importance of single-cell analyses. Microbiol. Rev. 1996, 60, 641–696. [Google Scholar] [PubMed]
- Wheeler, A.R.; Throndset, W.R.; Whelan, R.J.; Leach, A.M.; Zare, R.N.; Liao, Y.H.; Farrell, K.; Manger, I.D.; Daridon, A. Microfluidic device for single-cell analysis. Anal. Chem. 2003, 75, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.S.; Anderson, W.F.; Quake, S.R. Microfluidic single-cell mRNA isolation and analysis. Anal. Chem. 2006, 78, 3084–3089. [Google Scholar] [CrossRef] [PubMed]
- He, M.Y.; Edgar, J.S.; Jeffries, G.D.M.; Lorenz, R.M.; Shelby, J.P.; Chiu, D.T. Selective encapsulation of single cells and subcellular organelles into picoliter- and femtoliter-volume droplets. Anal. Chem. 2005, 77, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Huebner, A.; Srisa-Art, M.; Holt, D.; Abell, C.; Hollfelder, F.; Demello, A.J.; Edel, J.B. Quantitative detection of protein expression in single cells using droplet microfluidics. Chem. Commun. 2007, 2007, 1218–1220. [Google Scholar] [CrossRef] [PubMed]
- Mazutis, L.; Gilbert, J.; Ung, W.L.; Weitz, D.A.; Griffiths, A.D.; Heyman, J.A. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Byvank, T.; Chang, W.J.; Bharde, A.; Vieira, G.; Miller, B.L.; Chalmers, J.J.; Bashir, R.; Sooryakumar, R. On-chip magnetic separation and encapsulation of cells in droplets. Lab Chip 2013, 13, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Huebner, A.; Olguin, L.F.; Bratton, D.; Whyte, G.; Huck, W.T.S.; de Mello, A.J.; Edel, J.B.; Abell, C.; Hollfelder, F. Development of quantitative cell-based enzyme assays in microdroplets. Anal. Chem. 2008, 80, 3890–3896. [Google Scholar] [CrossRef] [PubMed]
- Baret, J.C.; Miller, O.J.; Taly, V.; Ryckelynck, M.; El-Harrak, A.; Frenz, L.; Rick, C.; Samuels, M.L.; Hutchison, J.B.; Agresti, J.J.; et al. Fluorescence-activated droplet sorting (FADS): Efficient microfluidic cell sorting based on enzymatic activity. Lab Chip 2009, 9, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Bae, C.Y.; Han, J.I.; Park, J.K. In situ analysis of heterogeneity in the lipid content of single green microalgae in alginate hydrogel microcapsules. Anal. Chem. 2013, 85, 8749–8756. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Jang, M.; Park, J.K. Rapid one-step purification of single-cells encapsulated in alginate microcapsules from oil to aqueous phase using a hydrophobic filter paper: Implications for single-cell experiments. Biotechnol. J. 2014, 9, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Edd, J.F.; Di Carlo, D.; Humphry, K.J.; Koster, S.; Irimia, D.; Weitz, D.A.; Toner, M. Controlled encapsulation of single-cells into monodisperse picolitre drops. Lab Chip 2008, 8, 1262–1264. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.R.; Chen, C.H.; Agresti, J.J.; Weitz, D.A. Beating poisson encapsulation statistics using close-packed ordering. Lab Chip 2009, 9, 2628–2631. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T.L. Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J. Appl. Phycol. 2012, 24, 989–1001. [Google Scholar] [CrossRef]
- Ahn, K.; Kerbage, C.; Hunt, T.P.; Westervelt, R.M.; Link, D.R.; Weitz, D.A. Dielectrophoretic manipulation of drops for high-speed microfluidic sorting devices. Appl. Phys. Lett. 2006, 88, 024104. [Google Scholar] [CrossRef]
- Zeng, S.J.; Li, B.W.; Su, X.O.; Qin, J.H.; Lin, B.C. Microvalve-actuated precise control of individual droplets in microfluidic devices. Lab Chip 2009, 9, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.; Abate, A.R.; Weitz, D.A.; Wixforth, A. Surface acoustic wave (SAW) directed droplet flow in microfluidics for PDMS devices. Lab Chip 2009, 9, 2625–2627. [Google Scholar] [CrossRef] [PubMed]
- Um, E.; Lee, S.G.; Park, J.K. Random breakup of microdroplets for single-cell encapsulation. Appl. Phys. Lett. 2010, 97, 153703. [Google Scholar] [CrossRef]
- Guillard, R.R. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Springer: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; von Maltzahn, G.; Zhang, L.; Schwartz, M.P.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Magnetic iron oxide nanoworms for tumor targeting and imaging. Adv. Mater. 2008, 20, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Park, J.K. Magnetic force-based multiplexed immunoassay using superparamagnetic nanoparticles in microfluidic channel. Lab Chip 2005, 5, 657–664. [Google Scholar] [PubMed]
- Xu, L.; Guo, C.; Wang, F.; Zheng, S.; Liu, C.Z. A simple and rapid harvesting method for microalgae by in situ magnetic separation. Bioresour. Technol. 2011, 102, 10047–10051. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Chieh, D.C.; Jalak, S.A.; Toh, P.Y.; Yasin, N.H.; Ng, B.W.; Ahmad, A.L. Rapid magnetophoretic separation of microalgae. Small 2012, 8, 1683–1692. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, Y.; Shen, F.; Hahn, Y.K.; Park, J.-H.; Park, J.-K. Magnetophoretic Sorting of Single Cell-Containing Microdroplets. Micromachines 2016, 7, 56. https://doi.org/10.3390/mi7040056
Jo Y, Shen F, Hahn YK, Park J-H, Park J-K. Magnetophoretic Sorting of Single Cell-Containing Microdroplets. Micromachines. 2016; 7(4):56. https://doi.org/10.3390/mi7040056
Chicago/Turabian StyleJo, Younggeun, Fengshan Shen, Young Ki Hahn, Ji-Ho Park, and Je-Kyun Park. 2016. "Magnetophoretic Sorting of Single Cell-Containing Microdroplets" Micromachines 7, no. 4: 56. https://doi.org/10.3390/mi7040056
APA StyleJo, Y., Shen, F., Hahn, Y. K., Park, J. -H., & Park, J. -K. (2016). Magnetophoretic Sorting of Single Cell-Containing Microdroplets. Micromachines, 7(4), 56. https://doi.org/10.3390/mi7040056