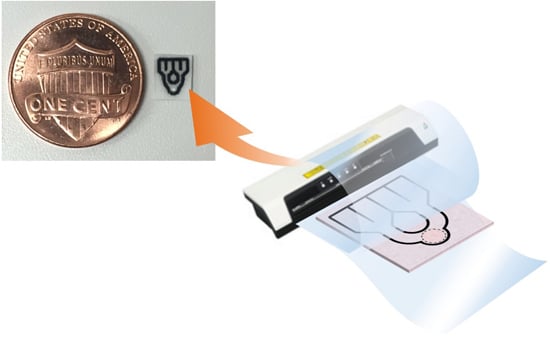
High-Resolution Microfluidic Paper-Based Analytical Devices for Sub-Microliter Sample Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Device Fabrication
2.3. Human Serum Albumin Assay
3. Results and Discussion
3.1. Heating Method of Melting Printed Wax into Paper Substrates
3.2. Device Design and Optimization
3.2.1. Influence of Cellulose Fiber Direction
3.2.2. Optimization of Flow Channel Barrier Width
3.2.3. Optimization of Flow Channel Width
3.2.4. Optimized µPAD Design
3.3. Human Serum Albumin (HSA) Assay
3.3.1. Colorimetric Assay with Sub-Microliter Sample Volume
3.3.2. Sample Volume Variation Tolerance of the Colorimetric Signal
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xia, Y.; Si, J.; Li, Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: A review. Biosens. Bioelectron. 2016, 77, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent Developments in Paper-Based Microfluidic Devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Paper-Based Inkjet-Printed Microfluidic Analytical Devices. Angew. Chem. Int. Ed. 2015, 54, 5294–5310. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Y.; Fu, J.-Z.; Wu, W.-B. Fabrication of paper-based microfluidic analysis devices: A review. RSC Adv. 2015, 5, 78109–78127. [Google Scholar] [CrossRef]
- Ahmed, S.; Bui, M.-P.N.; Abbas, A. Paper-based chemical and biological sensors: Engineering aspects. Biosens. Bioelectron. 2016, 77, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Fiorini, P.; Peeters, S.; Majeed, B.; Sterken, T.; Op de Beeck, M.; Yamashita, H. Sub-micro-liter electrochemical single-nucleotide-polymorphism setector for lab-on-chip system. Jpn. J. Appl. Phys. 2012, 51, 04DL02. [Google Scholar] [CrossRef]
- Huang, S.-B.; Lee, G.-B. Pneumatically driven micro-dispenser for sub-micro-liter pipetting. J. Micromech. Microeng. 2009, 19, 035027. [Google Scholar] [CrossRef]
- Li, L.; Boedicker, J.Q.; Ismagilov, R.F. Using a multijunction microfluidic device to inject substrate into an array of preformed plugs without cross-contamination: comparing theory and experiments. Anal. Chem. 2007, 79, 2756–2761. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Takaki, S.; Komuro, N.; Suzuki, K.; Citterio, D. An antibody-free microfluidic paper-based analytical device for the determination of tear fluid lactoferrin by fluorescence sensitization of Tb3+. Analyst 2014, 139, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Rajasingham, R.; Noubary, F.; Coonahan, E.; Schoeplein, R.; Baden, R.; Curry, M.; Afdhal, N.; Kumar, S.; Pollock, N.R. Performance of an Optimized Paper-Based Test for Rapid Visual Measurement of Alanine Aminotransferase (ALT) in Fingerstick and Venipuncture Samples. PLOS ONE 2015, 10, e0128118. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shi, W.; Jiang, L.; Qin, J.; Lin, B. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis 2009, 30, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-G.; Lee, S.-H.; Choi, C.-H.; Kim, J.; Lee, C.-S. Toward instrument-free digital measurements: A three-dimensional microfluidic device fabricated in a single sheet of paper by double-sided printing and lamination. Lab Chip 2015, 15, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Johnson, M.A. Measurement of total antioxidant capacity in sub-μL blood samples using craft paper-based analytical devices. RSC Adv. 2015, 5, 55633–55639. [Google Scholar] [CrossRef]
- Yeh, S.-H.; Chou, K.-H.; Yang, R.-J. Sample pre-concentration with high enrichment factors at a fixed location in paper-based microfluidic devices. Lab Chip 2016, 16, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Sariri, R.; Ghafoori, H. Tear proteins in health, disease, and contact lens wear. Biochem. Mosc. 2008, 73, 381–392. [Google Scholar] [CrossRef]
- Rantamäki, A.H.; Seppänen-Laakso, T.; Oresic, M.; Jauhiainen, M.; Holopainen, J.M. Human tear fluid lipidome: from composition to function. PLOS ONE 2011, 6, e19553. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Enomae, T. Paper substrate modification for rapid capillary flow in microfluidic paper-based analytical devices. RSC Adv. 2014, 4, 12867–12872. [Google Scholar] [CrossRef]
- Suzuki, Y. Theoretical analysis concerning the characteristics of a dye-binding method for determining serum protein based on protein error of pH indicator: effect of buffer concentration of the color reagent on the color development. Anal. Sci. 2005, 21, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Beuerman, R.W.; Foo, Y.; Liu, S.; Ang, L.P.; Tan, D.T. Characterisation of human tear proteins using high-resolution mass spectrometry. Ann. Acad. Med. Singap. 2006, 35, 400–407. [Google Scholar] [PubMed]







| Printer Set Wax Barrier Width (µm) | 200 | 250 | 300 | 350 |
|---|---|---|---|---|
| Number of functional barriers (n = 20) | 13/20 | 12/20 | 20/20 | 20/20 |
| Actual pictures |  |  |  |  |
| Printer Set Channel Width (µm) | 300 | 400 | 500 |
|---|---|---|---|
| Number of functional channels (n = 20) | 4/20 | 20/20 | 20/20 |
| Actual pictures |  |  |  |
| Sample Volume (µL) | 0.3 | 0.4 | 0.5 | 0.6 |
|---|---|---|---|---|
| Success rate (n = 5) | 0/5 | 1/5 | 5/5 | 5/5 |
| Actual pictures |  |  |  |  |
| Sample Volume (µL) | Slope | y-Intercept | R2 | Mean of Relative Standard Deviations |
|---|---|---|---|---|
| 0.7 | −4.21 | 166 | 0.979 | 2.08% |
| 0.8 | −4.22 | 164 | 0.956 | 2.40% |
| 0.9 | −4.01 | 163 | 0.931 | 1.78% |
| Average | −4.15 ± 0.12 | 164 ± 1.5 | - | - |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenda, K.; Ota, R.; Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. High-Resolution Microfluidic Paper-Based Analytical Devices for Sub-Microliter Sample Analysis. Micromachines 2016, 7, 80. https://doi.org/10.3390/mi7050080
Tenda K, Ota R, Yamada K, Henares TG, Suzuki K, Citterio D. High-Resolution Microfluidic Paper-Based Analytical Devices for Sub-Microliter Sample Analysis. Micromachines. 2016; 7(5):80. https://doi.org/10.3390/mi7050080
Chicago/Turabian StyleTenda, Keisuke, Riki Ota, Kentaro Yamada, Terence G. Henares, Koji Suzuki, and Daniel Citterio. 2016. "High-Resolution Microfluidic Paper-Based Analytical Devices for Sub-Microliter Sample Analysis" Micromachines 7, no. 5: 80. https://doi.org/10.3390/mi7050080
APA StyleTenda, K., Ota, R., Yamada, K., Henares, T. G., Suzuki, K., & Citterio, D. (2016). High-Resolution Microfluidic Paper-Based Analytical Devices for Sub-Microliter Sample Analysis. Micromachines, 7(5), 80. https://doi.org/10.3390/mi7050080