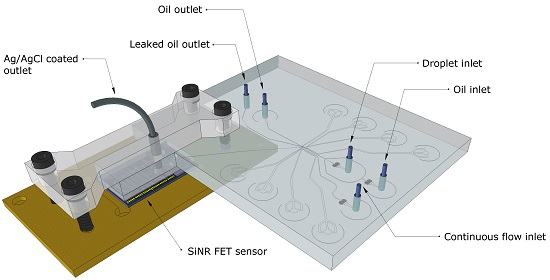
Integration of a Droplet-Based Microfluidic System and Silicon Nanoribbon FET Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silicon Nanoribbon FET Fabrication
2.2. Fabrication of the On-Chip Passivation and Microchannel Systems
2.3. Microfluidic Droplet Station Setup
2.4. Fabrication of Droplet Microfluidic Chips
2.5. System Integration
2.6. Characterization of SiNR FET Sensors
2.7. Operation of the Droplet Microfluidic Device
2.8. Analysis of Aqueous Droplets Using SiNR FET Sensors
3. Results
3.1. Electrical Characterization of Silicon Nanoribbon FET Sensors
3.2. Continuous Electric Field and pH Sensing
3.3. Effect of the Electric Field and the Pulse-Time
3.4. Pulse-Controlled Droplet Transfer and pH Detection
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Theberge, A.B.; Courtois, F.; Schaerli, Y.; Fischlechner, M.; Abell, C.; Hollfelder, F.; Huck, W.T.S. Microdroplets in microfluidics: An evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed. Engl. 2010, 49, 5846–5868. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Ismagilov, R.F. Millisecond Kinetics on a Microfluidic Chip Using Nanoliters of Reagents. J. Am. Chem. Soc. 2003, 125, 14613–14619. [Google Scholar] [CrossRef] [PubMed]
- Joensson, H.N.; Andersson Svahn, H. Droplet Microfluidics-A Tool for Single-Cell Analysis. Angew. Chem. Int. Ed. 2012, 51, 12176–12192. [Google Scholar] [CrossRef] [PubMed]
- Baret, J.-C.; Miller, O.J.; Taly, V.; Ryckelynck, M.; El-harrak, A.; Frenz, L.; Rick, C.; Samuels, M.L.; Hutchison, J.B.; Agresti, J.J.; et al. Fluorescence-activated droplet sorting ( FADS): Efficient microfluidic cell sorting based on enzymatic activity. Lab Chip 2009, 9, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Pekin, D.; Skhiri, Y.; Baret, J.-C.; Le Corre, D.; Mazutis, L.; Salem, C.B.; Millot, F.; El Harrak, A.; Hutchison, J.B.; Larson, J.W.; et al. Quantitative and sensitive detection of rare mutations using droplet-based microfluidics. Lab Chip 2011, 11, 2156–2166. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, S.L.; Joensson, H.N.; Svahn, H.A. Multiplex analysis of enzyme kinetics and inhibition by droplet microfluidics using picoinjectors. Lab Chip 2013, 13, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Kemna, E.W. M.; Segerink, L.I.; Wolbers, F.; Vermes, I.; van den Berg, A. Label-free, high-throughput, electrical detection of cells in droplets. Analyst 2013, 138, 4585–4592. [Google Scholar] [CrossRef] [PubMed]
- Isgor, P.K.; Marcali, M.; Keser, M.; Elbuken, C. Microfluidic droplet content detection using integrated capacitive sensors. Sens. Actuators B. Chem. 2015, 210, 669–675. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Chang, Y.; Sawtelle, S.D.; Wipf, M.; Duan, X.; Reed, M.A. Silicon Nanowire Field-Effect Transistors—A Versatile Class of Potentiometric Nanobiosensors. IEEE Access 2015, 3, 287–302. [Google Scholar] [CrossRef]
- Zhang, A.; Lieber, C.M. Nano-Bioelectronics. Chem. Rev. 2016, 116, 215–257. [Google Scholar] [CrossRef] [PubMed]
- Fennell, J.F.; Liu, S.F.; Azzarelli, J.M.; Weis, J.G.; Rochat, S.; Mirica, K.A.; Ravnsbæk, J.B.; Swager, T.M. Nanowire Chemical/Biological Sensors: Status and a Roadmap for the Future. Angew. Chem. Int. Ed. 2016, 55, 1266–1281. [Google Scholar] [CrossRef] [PubMed]
- Yates, D.E.; Levine, S.; Healy, T.W. Site-binding model of the electrical double layer at the oxide/water interface. J. Chem. Soc. 1974, 70, 1807. [Google Scholar] [CrossRef]
- Bousse, L.; De Rooij, N.F.; Bergveld, P. Operation of chemically sensitive field-effect sensors as a function of the insulator-electrolyte interface. IEEE Trans. Electron Devices 1983, 30, 1263–1270. [Google Scholar] [CrossRef]
- Mu, L.; Droujinine, I.A.; Rajan, N.K.; Sawtelle, S.D.; Reed, M. A Direct, Rapid, and Label-Free Detection of Enzyme–Substrate Interactions in Physiological Buffers Using CMOS-Compatible Nanoribbon Sensors. Nano Lett. 2014, 14, 5315–5322. [Google Scholar] [CrossRef] [PubMed]
- Fallah-Araghi, A.; Baret, J.-C.; Ryckelynck, M.; Griffiths, A.D. A completely in vitro ultrahigh-throughput droplet-based microfluidic screening system for protein engineering and directed evolution. Lab Chip 2012, 12, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Toumazou, C.; Shepherd, L.M.; Reed, S.C.; Chen, G.I.; Patel, A.; Garner, D.M.; Wang, C.A.; Ou, C.; Amin-desai, K.; Athanasiou, P.; et al. Simultaneous DNA amplification and detection using a pH-sensing semiconductor system. Nat. Methods 2013, 10, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Rane, T.D.; Chen, L.; Zec, H.C.; Wang, T. Microfluidic continuous flow digital loop-mediated isothermal amplification (LAMP). Lab Chip 2014, 15, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yao, H.; Tzang, C.H.; Zhu, J.; Yang, M.; Lee, S.T. Silicon nanowires for high-sensitivity glucose detection. Appl. Phys. Lett. 2006, 88, 213104. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Hong, M.; Erramilli, S.; Mohanty, P. Surface-modified silicon nano-channel for urea sensing. Sens. Actuators B Chem. 2008, 133, 593–598. [Google Scholar] [CrossRef]
- Craighead, H. Future lab-on-a-chip technologies for interrogating individual molecules. Nature 2006, 442, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Credo, G.M.; Su, X.; Wu, K.; Elibol, O.H.; Liu, D.J.; Reddy, B.; Tsai, T.-W.; Dorvel, B.R.; Daniels, J.S.; Bashir, R.; et al. Label-free electrical detection of pyrophosphate generated from DNA polymerase reactions on field-effect devices. Analyst 2012, 137, 1351. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Liao, Y.-C.; Tsai, Y.-T.; Yeh, H.-I.; Wu, C.-C. Multiple Silicon Nanowires with Enzymatic Modification for Measuring Glucose Concentration. Micromachines 2015, 6, 1135–1142. [Google Scholar] [CrossRef]
- Song, H.; Chen, D.L.; Ismagilov, R.F. Reactions in droplets in microfluidic channels. Angew. Chem. Int. Ed. Engl. 2006, 45, 7336–7356. [Google Scholar] [CrossRef] [PubMed]
- Han, Y. Label-free detection of biomolecules by a f ield-effect transistor microarray biosensor with bio-functionalized gate surfaces. Available online: http://zb0035.zb.kfa-juelich.de/record/52661/files/Juel_4227_Han.pdf (accessed on 2 August 2016).
- Matsumoto, A.; Miyahara, Y. Current and emerging challenges of field effect transistor based bio-sensing. Nanoscale 2013, 5, 10702–10718. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, R.; Jokilaakso, N.; Schmidt, T.; Björk, P.; Karlström, A.E.; Linnros, J. Effect of microwave-assisted silanization on sensing properties of silicon nanoribbon FETs. Sens. Actuators B Chem. 2015, 209, 586–595. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Williams, K.R.; Gupta, K.; Wasilik, M. Etch rates for micromachining processing-part II. J. Microelectromech. Syst. 2003, 12, 761–778. [Google Scholar] [CrossRef]
- Topkar, A.; Lal, R. Effect of electrolyte exposure on silicon dioxide in electrolyte-oxide-semiconductor structures. Thin Solid Films 1993, 232, 265–270. [Google Scholar] [CrossRef]








© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afrasiabi, R.; Soderberg, L.M.; Joensson, H.N.; Björk, P.; Andersson Svahn, H.; Linnros, J. Integration of a Droplet-Based Microfluidic System and Silicon Nanoribbon FET Sensor. Micromachines 2016, 7, 134. https://doi.org/10.3390/mi7080134
Afrasiabi R, Soderberg LM, Joensson HN, Björk P, Andersson Svahn H, Linnros J. Integration of a Droplet-Based Microfluidic System and Silicon Nanoribbon FET Sensor. Micromachines. 2016; 7(8):134. https://doi.org/10.3390/mi7080134
Chicago/Turabian StyleAfrasiabi, Roodabeh, Lovisa M. Soderberg, Haakan N. Joensson, Per Björk, Helene Andersson Svahn, and Jan Linnros. 2016. "Integration of a Droplet-Based Microfluidic System and Silicon Nanoribbon FET Sensor" Micromachines 7, no. 8: 134. https://doi.org/10.3390/mi7080134
APA StyleAfrasiabi, R., Soderberg, L. M., Joensson, H. N., Björk, P., Andersson Svahn, H., & Linnros, J. (2016). Integration of a Droplet-Based Microfluidic System and Silicon Nanoribbon FET Sensor. Micromachines, 7(8), 134. https://doi.org/10.3390/mi7080134