Enhanced Throughput for Electrokinetic Manipulation of Particles and Cells in a Stacked Microfluidic Device
Abstract
:1. Introduction
2. Experiment
2.1. Microfluidic Device Fabrication
2.2. Particle and Cell Solutions Preparation
2.3. Particle/Cell Control and Visualization
3. Theory
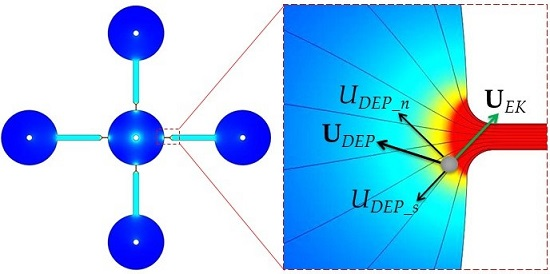
3.1. Electrokinetic Particle and Cell Manipulation via rDEP
3.2. Numerical Simulation
4. Results and Discussion
4.1. Electrokinetic Parallel Concentration of 5-μm Polystyrene Particles
4.2. Electrokinetic Parallel Separation of 5-μm and 3-μm Polystyrene Particles
4.3. Electrokinetic Parallel Concentration of Yeast Cells
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bruin, G.J.M. Recent developments in electrokinetically driven analysis on microfabricated devices. Electrophoresis 2000, 21, 3931–3951. [Google Scholar] [PubMed]
- Wong, P.K.; Wang, T.; Deval, J.H.; Ho, C.M. Electrokinetics in micro devices for biotechnology applications. IEEE/ASME Trans. Mechatron. 2004, 9, 366–376. [Google Scholar]
- Chang, H.C.; Yeo, L.Y. Electrokinetically Driven Microfluidics and Nanofluidics; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Li, D. Electrokinetics in Microfluidics; Elsevier Academic Press: Burlington, MA, USA, 2004. [Google Scholar]
- Masliyah, J.H.; Bhattacharjee, S. Electrokinetic and Colloid Transport Phenomena; Wiley-Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- Hunter, R.J. Zeta Potential in Colloid Science; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Lyklemma, J. Fundamentals of Interface and Colloid Science; Academic Press: New York, NY, USA, 1991. [Google Scholar]
- Whitesides, G.M.; Stroock, A.D. Flexible methods for microfluidics. Phys. Today 2001, 54, 42–48. [Google Scholar]
- Kang, Y.; Li, D. Electrokinetic motion of particles and cells in microchannels. Microfluid. Nanofluid. 2009, 6, 431–460. [Google Scholar]
- Qian, S.; Ai, Y. Electrokinetic Particle Transport in Micro-/Nanofluidics: Direct Numerical Simulation Analysis; CRC Press: Cleveland, OH, USA, 2012. [Google Scholar]
- Probstein, R.F. Physicochemical Hydrodynamics, 2nd ed.; John Willey and Sons: New York, NY, USA, 1994. [Google Scholar]
- Kirby, B.J. Micro and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Xuan, X. Joule heating in electrokinetic flow. Electrophoresis 2008, 29, 33–43. [Google Scholar] [PubMed]
- Cetin, B.; Li, D.Q. Effect of Joule heating on electrokinetic transport. Electrophoresis 2008, 29, 994–1005. [Google Scholar] [PubMed]
- Lee, R.C. Cell injury by electric forces. Ann. N. Y. Acad. Sci. 2005, 1066, 85–91. [Google Scholar] [PubMed]
- Voldman, J. Electrical forces for microscale cell manipulation. Annu. Rev. Biomed. Eng. 2006, 8, 425–454. [Google Scholar] [PubMed]
- Saucedo-Espinosa, M.A.; Lapizco-Encinas, B.H. Refinement of current monitoring methodology for electroosmotic flow assessment under low ionic strength conditions. Biomicrofluidics 2016, 10, 033104. [Google Scholar] [PubMed]
- Yung, C.W.; Fiering, J.; Mueller, A.J.; Ingber, D.E. Micromagnetic–microfluidic blood cleansing device. Lab Chip 2009, 9, 1171–1177. [Google Scholar] [PubMed]
- Choi, S.; Ku, T.; Song, S.; Choi, C.; Park, J.K. Hydrophoretic high-throughput selection of platelets in physiologicalshear-stress range. Lab Chip 2011, 11, 413–418. [Google Scholar] [PubMed]
- Hur, S.C.; Mach, A.J.; Di Carlo, D. High-throughput size-based rare cell enrichment using microscale vortices. Biomicrofluidics 2011, 5, 022206. [Google Scholar]
- Hyun, K.A.; Kwon, K.; Han, H.; Kim, S.; Jung, H. Microfluidic flow fractionation device for label-free isolation of circulating tumor cells (CTCs) from breast cancer patients. Biosens. Bioelectron. 2013, 40, 206–212. [Google Scholar] [PubMed]
- Kim, S.; Kim, J.; Kim, D.; Han, S.; Weitz, D. Enhanced-throughput production of polymersomes using a parallelized capillary microfluidic device. Microfluid. Nanofluid. 2013, 14, 509–514. [Google Scholar]
- Zhang, J.; Yan, S.; Li, W.; Alicia, G.; Nguyen, N.T. High throughput extraction of plasma using a secondary flow-aided inertial microfluidic device. RSC Adv. 2014, 4, 33149–33159. [Google Scholar]
- Yu, Z.T.F.; Guan, H.; Cheung, M.K.; McHugh, W.M.; Cornell, T.T.; Shanley, T.P.; Kurabayashi, K.; Fu, J. Rapid, automated, parallel quantitative immunoassays using highly integrated microfluidics and AlphaLISA. Sci. Rep. 2015, 11339. [Google Scholar] [CrossRef]
- Patel, S.; Showers, D.; Vedantam, P.; Tzeng, T.; Qian, S.; Xuan, X. Microfluidic separation of live and dead yeast cells using reservoir-based dielectrophoresis (rDEP). Biomicrofluidics 2012, 6, 034102. [Google Scholar]
- Patel, S.; Qian, S.; Xuan, X. Reservoir-based dielectrophoresis (rDEP) for microfluidic particle separation by charge. Electrophoresis 2013, 34, 961–968. [Google Scholar] [PubMed]
- Zhu, J.; Hu, G.; Xuan, X. Electrokinetic particle entry into microchannels. Electrophoresis 2012, 33, 916–922. [Google Scholar] [PubMed]
- Ermolina, I.; Morgan, H. The electrokinetic properties of latex particles: Comparison of electrophoresis and dielectrophoresis. J. Colloid Interface Sci. 2005, 285, 419–428. [Google Scholar] [PubMed]
- Morgan, H.; Green, N.G. AC Electrokinetics: Colloids and Nanoparticles; Research Studies Press: Hertfordshire, UK, 2002. [Google Scholar]
- Cummings, E.B.; Griffiths, S.K.; Nilson, R.H.; Paul, P.H. Conditions for similitude between the fluid velocity and electric field in electroosmotic flow. Anal. Chem. 2000, 72, 2526–2532. [Google Scholar] [PubMed]
- Zhu, J.; Xuan, X. Dielectrophoretic focusing of particles in a microchannel constriction using DC-biased AC electric fields. Electrophoresis 2009, 30, 2668–2675. [Google Scholar] [PubMed]
- Kang, K.; Kang, Y.; Xuan, X.; Li, D. Continuous separation of microparticles by size with DC-dielectrophoresis. Electrophoresis 2006, 27, 694–702. [Google Scholar] [PubMed]
- Zhu, J.; Canter, R.C.; Keten, G.; Vedantam, P.; Tzeng, T.; Xuan, X. Continuous flow separation of particles and cells in a serpentine microchannel via curvature-induced dielectrophoresis. Microfluid. Nanofluid. 2011, 11, 743–752. [Google Scholar]
- Church, C.; Zhu, J.; Wang, G.; Tzeng, T.J.; Xuan, X. Electrokinetic focusing and filtration of cells in a serpentine microchannel. Biomicrofluidics 2009, 3, 044109. [Google Scholar]





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Patel, S.H.; Johnson, M.; Kale, A.; Raval, Y.; Tzeng, T.-R.; Xuan, X. Enhanced Throughput for Electrokinetic Manipulation of Particles and Cells in a Stacked Microfluidic Device. Micromachines 2016, 7, 156. https://doi.org/10.3390/mi7090156
Zhu L, Patel SH, Johnson M, Kale A, Raval Y, Tzeng T-R, Xuan X. Enhanced Throughput for Electrokinetic Manipulation of Particles and Cells in a Stacked Microfluidic Device. Micromachines. 2016; 7(9):156. https://doi.org/10.3390/mi7090156
Chicago/Turabian StyleZhu, Lin, Saurin H. Patel, Mark Johnson, Akshay Kale, Yash Raval, Tzuen-Rong Tzeng, and Xiangchun Xuan. 2016. "Enhanced Throughput for Electrokinetic Manipulation of Particles and Cells in a Stacked Microfluidic Device" Micromachines 7, no. 9: 156. https://doi.org/10.3390/mi7090156
APA StyleZhu, L., Patel, S. H., Johnson, M., Kale, A., Raval, Y., Tzeng, T. -R., & Xuan, X. (2016). Enhanced Throughput for Electrokinetic Manipulation of Particles and Cells in a Stacked Microfluidic Device. Micromachines, 7(9), 156. https://doi.org/10.3390/mi7090156