Aerogels for Optofluidic Waveguides
Abstract
:1. Introduction
2. Aerogels
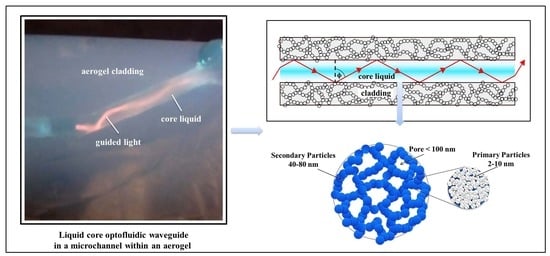
3. Aerogel-Based Optofluidic Waveguides
4. Preparation of Aerogel Waveguides
4.1. Synthesis of Aerogels
4.2. Surface Modification of Aerogels
4.3. Channel Formation in Aerogel Monoliths
5. Characterization of Aerogel-Based Waveguides
6. Applications of Aerogel-Based Optofluidic Waveguides
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Psaltis, D.; Quake, S.R.; Yang, C. Developing optofluidic technology through the fusion of microfluidics and optics. Nature 2006, 442, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Monat, C.; Domachuk, P.; Eggleton, B.J. Integrated optofluidics: A new river of light. Nat. Photon. 2007, 1, 106–114. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M. Optofluidic microsystems for chemical and biological analysis. Nat. Photon. 2011, 5, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; Sinton, D.; Psaltis, D. Optofluidics for energy applications. Nat. Photon. 2011, 5, 583–590. [Google Scholar] [CrossRef]
- Schmidt, H.; Hawkins, A.R. Optofluidic waveguides: I. Concepts and implementations. Microfluid. Nanofluid. 2008, 4, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Rowland, K.J.; Afshar, V.S.; Stolyarov, A.; Fink, Y.; Monro, T.M. Bragg waveguides with low-index liquid cores. Opt. Express 2012, 20, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Pone, E.; Dubois, C.; Guo, N.; Gao, Y.; Dupuis, A.; Boismenu, F.; Lacroix, S.; Skorobogatiy, M. Drawing of the hollow all-polymer Bragg fibers. Opt. Express 2006, 14, 5838–5852. [Google Scholar] [PubMed]
- Cubillas, A.M.; Unterkofler, S.; Euser, T.G.; Etzold, B.J.M.; Jones, A.C.; Sadler, P.J.; Wasserscheid, P.; Russell, P.S.J. Photonic crystal fibres for chemical sensing and photochemistry. Chem. Soc. Rev. 2013, 42, 8629–8648. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Campopiano, S.; Zeni, L.; Sarro, P.M. ARROW optical waveguides based sensors. Sens. Actuators B: Chem. 2004, 100, 143–146. [Google Scholar] [CrossRef]
- Testa, G.; Persichetti, G.; Bernini, R. Liquid core ARROW waveguides: A promising photonic structure for integrated optofluidic microsensors. Micromachines 2016, 7, 47. [Google Scholar] [CrossRef]
- Risk, W.P.; Kim, H.C.; Miller, R.D.; Temkin, H.; Gangopadhyay, S. Optical waveguides with an aqueous core and a low-index nanoporous cladding. Opt. Express 2004, 12, 6446–6455. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.R.; Schmidt, H. Optofluidic waveguides: II. Fabrication and structures. Microfluid. Nanofluid. 2008, 4, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Uranus, H.P. Guiding Light by and Beyond the Total Internal Reflection Mechanism. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2005. [Google Scholar]
- Freeberg, B. Liquid Fiber Optics. Available online: http://www.aapt.org/Programs/contests/winnersfull.cfm?id=2687&theyear=2011 (accessed on 25 February 2017).
- Schelle, B.; Dress, P.; Franke, H.; Klein, K.F.; Slupek, J. Physical characterization of lightguide capillary cells. J. Phys. D Appl. Phys. 1999, 32, 3157–3163. [Google Scholar] [CrossRef]
- Dress, P.; Franke, H. An Optical Fiber with a Liquid H2O Core; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 1996; Volume 2686, pp. 157–163. [Google Scholar]
- Cai, H.; Parks, J.W.; Wall, T.A.; Stott, M.A.; Stambaugh, A.; Alfson, K.; Griffiths, A.; Mathies, R.A.; Carrion, R.; Patterson, J.L.; et al. Optofluidic analysis system for amplification-free, direct detection of Ebola infection. Sci. Rep. 2015, 5, 14494. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, K.S.; Im, S.; Destgeer, G.; Ha, B.H.; Park, J.; Sung, H.J. Photosynthesis of cyanobacteria in a miniaturized optofluidic waveguide platform. RSC Adv. 2016, 6, 11081–11087. [Google Scholar] [CrossRef]
- Yalizay, B.; Morova, Y.; Dincer, K.; Ozbakir, Y.; Jonas, A.; Erkey, C.; Kiraz, A.; Akturk, S. Versatile liquid-core optofluidic waveguides fabricated in hydrophobic silica aerogels by femtosecond-laser ablation. Opt. Mater. 2015, 47, 478–483. [Google Scholar] [CrossRef]
- Cho, S.H.; Godin, J.; Lo, Y.H. Optofluidic Waveguides in Teflon AF-Coated PDMS Microfluidic Channels. IEEE Photon. Technol. Lett. 2009, 21, 1057–1059. [Google Scholar]
- Datta, A.; Eom, I.Y.; Dhar, A.; Kuban, P.; Manor, R.; Ahmad, I.; Gangopadhyay, S.; Dallas, T.; Holtz, M.; Temkin, F.; et al. Microfabrication and characterization of Teflon AF-coated liquid core waveguide channels in silicon. IEEE Sens. J. 2003, 3, 788–795. [Google Scholar] [CrossRef]
- Wu, C.W.; Gong, G.C. Fabrication of PDMS-based nitrite sensors using Teflon AF coating microchannels. IEEE Sens. J. 2008, 8, 465–469. [Google Scholar] [CrossRef]
- Eris, G.; Sanli, D.; Ulker, Z.; Bozbag, S.E.; Jonás, A.; Kiraz, A.; Erkey, C. Three-dimensional optofluidic waveguides in hydrophobic silica aerogels via supercritical fluid processing. J. Supercrit. Fluids 2013, 73, 28–33. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U. Aerogels—Airy materials: Chemistry, structure, and properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Xiao, L.; Birks, T.A. Optofluidic microchannels in aerogel. Opt. Lett. 2011, 36, 3275–3277. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Grogan, M.D.; Leon-Saval, S.G.; Williams, R.; England, R.; Wadsworth, W.J.; Birks, T.A. Tapered fibers embedded in silica aerogel. Opt. Lett. 2009, 34, 2724–2726. [Google Scholar] [CrossRef] [PubMed]
- Yalizay, B.; Morova, Y.; Ozbakir, Y.; Jonas, A.; Erkey, C.; Kiraz, A.; Akturk, S. Optofluidic waveguides written in hydrophobic silica aerogels with a femtosecond laser. Proc. SPIE 2015, 9365, 936518. [Google Scholar]
- Sprehn, G.A.; Hrubesh, L.W.; Poco, J.F.; Sandler, P.H. Aerogel-Clad Optical Fiber. U.S. Patent 5,684,907, 4 November 1997. [Google Scholar]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A special material or a new state of matter: A review and reconsideration of the aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef]
- Fricke, J. Aerogels—Highly tenuous solids with fascinating properties. J. Non-Cryst. Solids 1988, 100, 169–173. [Google Scholar] [CrossRef]
- Handbook, A.; Aegerter, N. Leventis and MM Koebel; Springer: New York, NY, USA, 2011. [Google Scholar]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel based systems. J. Controll. Release 2014, 177, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhang, Y.; Zhang, L.; Miao, Y.-E.; Fan, W.; Liu, T. Polymer/carbon-based hybrid aerogels: preparation, properties and applications. Materials 2015, 8, 6828–6848. [Google Scholar] [CrossRef]
- Zhang, X.X.; Su, W.M.; Lin, M.Y.; Miao, X.; Ye, L.Q.; Yang, W.B.; Jiang, B. Non-supercritical drying sol-gel preparation of superhydrophobic aerogel ORMOSIL thin films with controlled refractive index. J. Sol-Gel Sci. Technol. 2015, 74, 594–602. [Google Scholar] [CrossRef]
- Hrubesh, L.W. Aerogel applications. J. Non-Cryst. Solids 1998, 225, 335–342. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring mechanical properties of aerogels for aerospace applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Bellunato, T.; Calvi, M.; Matteuzzi, C.; Musy, M.; Perego, D.L.; Storaci, B. Refractive index of silica aerogel: Uniformity and dispersion law. Nucl. Instrum. Methods Phys. Res. Sect. A 2008, 595, 183–186. [Google Scholar] [CrossRef]
- Tabata, M.; Adachi, I.; Kawai, H.; Kubo, M.; Sato, T. Recent progress in silica aerogel Cherenkov radiator. Phys. Proced. 2012, 37, 642–649. [Google Scholar] [CrossRef]
- Adachi, I.; Sumiyoshi, T.; Hayashi, K.; Iida, N.; Enomoto, R.; Tsukada, K.; Suda, R.; Matsumoto, S.; Natori, K.; Yokoyama, M.; et al. Study of a threshold Cherenkov counter based on silica aerogels with low refractive-indexes. Nucl. Instrum. Methods Phys. Res. Sect. A 1995, 355, 390–398. [Google Scholar] [CrossRef]
- Nappi, E. Aerogel and its applications to RICH detectors. Nucl. Phys. B 1998, 270–276. [Google Scholar] [CrossRef]
- Cantin, M.; Casse, M.; Koch, L.; Jouan, R.; Mestreau, P.; Roussel, D.; Bonnin, F.; Moutel, J.; Teichner, S.J. Silica aerogels used as Cherenkov radiators. Nucl. Instrum. Methods 1974, 118, 177–182. [Google Scholar] [CrossRef]
- Pajonk, G.M. Some applications of silica aerogels. Colloid Polym. Sci. 2003, 281, 637–651. [Google Scholar] [CrossRef]
- Chen, Q.; Long, D.; Chen, L.; Liu, X.; Liang, X.; Qiao, W.; Ling, L. Synthesis of ultrahigh-pore-volume carbon aerogels through a “reinforced-concrete” modified sol–gel process. J. Non-Cryst. Solids 2011, 357, 232–235. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and properties of resorcinol–formaldehyde organic and carbon gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Demilecamps, A.; Zhang, Y.; Brunner, S.; Huber, L.; Tingaut, P.; Rigacci, A.; Budtova, T.; Koebel, M.M. Strong, Thermally superinsulating biopolymer–silica aerogel hybrids by cogelation of silicic acid with pectin. Angew. Chem. Int. Ed. 2015, 54, 14282–14286. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, B.T.; Ragothaman, M.; Kalirajan, C.; Palanisamy, T. Conducting collagen-polypyrrole hybrid aerogels made from animal skin waste. RSC Adv. 2016, 6, 63071–63077. [Google Scholar] [CrossRef]
- Dong, W.; Rhine, W.; White, S. Polyimide-silica hybrid aerogels with high mechanical strength for thermal insulation applications. MRS Proc. 2011, 1306, mrsf10-1306-bb03-03. [Google Scholar] [CrossRef]
- Yang, Q.; Tan, X.; Wang, S.; Zhang, J.; Chen, L.; Zhang, J.-P.; Su, C.-Y. Porous organic–inorganic hybrid aerogels based on bridging acetylacetonate. Microporous Mesoporous Mater. 2014, 187, 108–113. [Google Scholar] [CrossRef]
- Yim, T.-J.; Kim, S.Y.; Yoo, K.-P. Fabrication and thermophysical characterization of nano-porous silica-polyurethane hybrid aerogel by sol-gel processing and supercritical solvent drying technique. Korean J. Chem. Eng. 2002, 19, 159–166. [Google Scholar] [CrossRef]
- Thapliyal, P.C.; Singh, K. Aerogels as promising thermal insulating materials: An overview. J. Mater. 2014, 2014, 10. [Google Scholar] [CrossRef]
- Ülker, Z.; Sanli, D.; Erkey, C. Applications of Aerogels and Their Composites in Energy-Related Technologies-Chapter 8. In Supercritical Fluid Technology for Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Jelle, B.P. Traditional, state-of-the-art and future thermal building insulation materials and solutions—Properties, requirements and possibilities. Energy Build. 2011, 43, 2549–2563. [Google Scholar] [CrossRef]
- Xiao, L.; Grogan, M.D.W.; Wadsworth, W.J.; England, R.; Birks, T.A. Stable low-loss optical nanofibres embedded in hydrophobic aerogel. Opt. Express 2011, 19, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Tamon, H.; Kitamura, T.; Okazaki, M. Preparation of silica aerogel from TEOS. J. Colloid Interface Sci. 1998, 197, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Beck, A.; Korner, W.; Scheller, H.; Fricke, J. Density and refractive index of silica aerogels after low- and high-temperature supercritical drying and thermal treatment. J. Phys. D: Appl. Phys. 1994, 27, 414. [Google Scholar] [CrossRef]
- Özbakır, Y.; Erkey, C. Experimental and theoretical investigation of supercritical drying of silica alcogels. J. Supercrit. Fluids 2015, 98, 153–166. [Google Scholar] [CrossRef]
- Özbakır, Y.; Ulker, Z.; Erkey, C. Monolithic composites of silica aerogel with poly (methyl vinyl ether) and the effect of polymer on supercritical drying. J. Supercrit. Fluids 2015, 105, 108–118. [Google Scholar] [CrossRef]
- Cheng, H.; Xue, H.; Hong, C.; Zhang, X. Characterization, thermal and mechanical properties and hydrophobicity of resorcinol-furfural/silicone hybrid aerogels synthesized by ambient-pressure drying. RSC Adv. 2016, 6, 75793–75804. [Google Scholar] [CrossRef]
- Alnaief, M.; Smirnova, I. Effect of surface functionalization of silica aerogel on their adsorptive and release properties. J. Non-Cryst. Solids 2010, 356, 1644–1649. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Amphiphilic superabsorbent cellulose nanofibril aerogels. J. Mater. Chemistry A 2014, 2, 6337–6342. [Google Scholar] [CrossRef]
- Wang, S.; Peng, X.; Zhong, L.; Tan, J.; Jing, S.; Cao, X.; Chen, W.; Liu, C.; Sun, R. An ultralight, elastic, cost-effective, and highly recyclable superabsorbent from microfibrillated cellulose fibers for oil spillage cleanup. J. Mater. Chemistry A 2015, 3, 8772–8781. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, P.; Wang, M.; Zhao, H.; Yang, J.; Xu, F. Sustainable, reusable, and superhydrophobic aerogels from microfibrillated cellulose for highly effective oil/water separation. ACS Sustain. Chem. Eng. 2016, 4, 6409–6416. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Kulkarni, M.M.; Amalnerkar, D.P.; Seth, T. Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor. J. Non-Cryst. Solids 2003, 330, 187–195. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kim, S.-Y.; Yoo, K.-P. Low-density, hydrophobic aerogels. J. Non-Cryst. Solids 1995, 186, 18–22. [Google Scholar] [CrossRef]
- Kartal, A.M.; Erkey, C. Surface modification of silica aerogels by hexamethyldisilazane–carbon dioxide mixtures and their phase behavior. J. Supercrit. Fluids 2010, 53, 115–120. [Google Scholar] [CrossRef]
- Smirnova, I.; Mamic, J.; Arlt, W. Adsorption of drugs on silica aerogels. Langmuir 2003, 19, 8521–8525. [Google Scholar] [CrossRef]
- Sun, J.; Longtin, J.P.; Norris, P.M. Ultrafast laser micromachining of silica aerogels. J. Non-Cryst. Solids 2001, 281, 39–47. [Google Scholar] [CrossRef]
- Bian, Q.M.; Chen, S.Y.; Kim, B.T.; Leventis, N.; Lu, H.B.; Chang, Z.H.; Lei, S.T. Micromachining of polyurea aerogel using femtosecond laser pulses. J. Non-Cryst. Solids 2011, 357, 186–193. [Google Scholar] [CrossRef]
- Akimov, Y.K.; Zrelov, V.P.; Puzynin, A.I.; Filin, S.V.; Filippov, A.I.; Sheinkman, V.A. An aerogel threshold Cerenkov counter. Instrum. Exp. Tech. 2002, 45, 634–639. [Google Scholar] [CrossRef]
- Henning, S.; Svensson, L. Production of silica aerogel. Phys. Scr. 1981, 23, 697. [Google Scholar] [CrossRef]
- Tabata, M.; Adachi, I.; Ishii, Y.; Kawai, H.; Sumiyoshi, T.; Yokogawa, H. Development of transparent silica aerogel over a wide range of densities. Nucl. Instrum. Methods Phys. Res. Sect. A 2010, 623, 339–341. [Google Scholar] [CrossRef]
- Pekala, R.W.; Alviso, C.T.; LeMay, J.D. Organic aerogels: Microstructural dependence of mechanical properties in compression. J. Non-Cryst. Solids 1990, 125, 67–75. [Google Scholar] [CrossRef]
- Woignier, T.; Reynes, J.; Hafidi Alaoui, A.; Beurroies, I.; Phalippou, J. Different kinds of structure in aerogels: Relationships with the mechanical properties. J. Non-Cryst. Solids 1998, 241, 45–52. [Google Scholar] [CrossRef]
- Worsley, M.A.; Kucheyev, S.O.; Satcher, J.H., Jr.; Hamza, A.V.; Baumann, T.F. Mechanically robust and electrically conductive carbon nanotube foams. Appl. Phys. Lett. 2009, 94, 073115. [Google Scholar] [CrossRef]
- Woignier, T.; Primera, J.; Alaoui, A.; Etienne, P.; Despestis, F.; Calas-Etienne, S. Mechanical properties and brittle behavior of silica aerogels. Gels 2015, 1, 256. [Google Scholar] [CrossRef]
- Buzykaev, A.; Danilyuk, A.; Ganzhur, S.; Gorodetskaya, T.; Kravchenko, E.; Onuchin, A.; Vorobiov, A. Aerogels with high optical parameters for Cherenkov counters. Nucl. Instrum. Methods Phys. Res. Sect. A 1996, 379, 465–467. [Google Scholar] [CrossRef]
- Kharzheev, Y.N. Use of silica aerogels in Cherenkov counters. Phys. Part. Nucl. 2008, 39, 107–135. [Google Scholar] [CrossRef]
- Fu, T.; Tang, J.; Chen, K.; Zhang, F. Visible, near-infrared and infrared optical properties of silica aerogels. Infrared Phys. Technol. 2015, 71, 121–126. [Google Scholar] [CrossRef]
- Pajonk, G.M. Transparent silica aerogels. J. Non-Cryst. Solids 1998, 225, 307–314. [Google Scholar] [CrossRef]
- Beck, A.; Gelsen, O.; Wang, P.; Fricke, J. Light scattering for structural investigations of silica aerogels and alcogels. J. Phys. Colloq. 1989, 50, C4-203–C4-208. [Google Scholar] [CrossRef]
- Jonas, A.; Yalizay, B.; Akturk, S.; Kiraz, A. Free-standing optofluidic waveguides formed on patterned superhydrophobic surfaces. Appl. Phys. Lett. 2014, 104, 091123. [Google Scholar] [CrossRef]
- Manor, R.; Datta, A.; Ahmad, I.; Holtz, M.; Gangopadhyay, S.; Dallas, T. Microfabrication and characterization of liquid core waveguide glass channels coated with Teflon AF. IEEE Sens. J. 2003, 3, 687–692. [Google Scholar] [CrossRef]
- Huang, G.W.; Chen, C.T. Microfluidic chips for guiding lights through micro polymeric channels injected with photopolymer liquids. IET Micro Nano Letters 2012, 7, 1080–1083. [Google Scholar] [CrossRef]


















© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özbakır, Y.; Jonas, A.; Kiraz, A.; Erkey, C. Aerogels for Optofluidic Waveguides. Micromachines 2017, 8, 98. https://doi.org/10.3390/mi8040098
Özbakır Y, Jonas A, Kiraz A, Erkey C. Aerogels for Optofluidic Waveguides. Micromachines. 2017; 8(4):98. https://doi.org/10.3390/mi8040098
Chicago/Turabian StyleÖzbakır, Yaprak, Alexandr Jonas, Alper Kiraz, and Can Erkey. 2017. "Aerogels for Optofluidic Waveguides" Micromachines 8, no. 4: 98. https://doi.org/10.3390/mi8040098
APA StyleÖzbakır, Y., Jonas, A., Kiraz, A., & Erkey, C. (2017). Aerogels for Optofluidic Waveguides. Micromachines, 8(4), 98. https://doi.org/10.3390/mi8040098