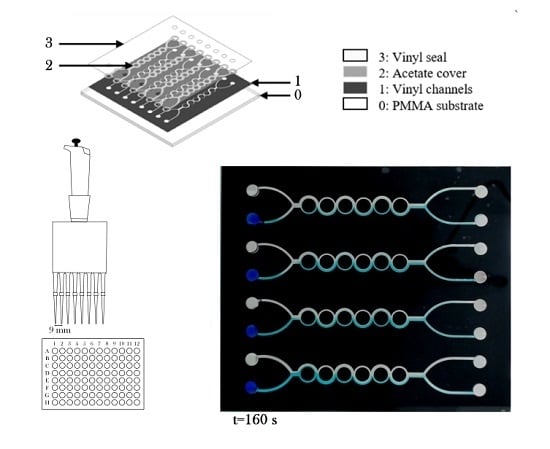
Rapid Fabrication of Disposable Micromixing Arrays Using Xurography and Laser Ablation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Xurography Setup
2.2. Laser Ablation Setup
2.3. Array Characterization
2.4. Micromixing Characterization
3. Results and Discussion
4. Conclusions and Future Work
- The dimensional accuracy of xurography was shown to be better for xurography than laser ablation for the ASAR micromixing array. Compared to xurography, the deployment of the laser ablation as a manufacturing tool in the POC setting underwent several disadvantages such as the requirement to adjust the setup parameters regarding the optical properties of the material and the additional health and security considerations for the laser processing of materials.
- Assessments of both the rapid manufacture technologies were successfully employed to produce low-cost microfluidic device arrays with deviational errors below 10% under certain setup conditions for xurography and laser ablation.
- Small differences in the dimensional errors among different ASAR micromixer members suggests that it is possible to scale-up further the size of the array.
- The proposed four element micromixer array design was successfully coupled with a standardized multichannel micropipette for micromixing simultaneously eight samples of dye with mixing performance up to 65%.
- The proposed design interfaces standardized dispensing (handheld micropipette) and sampling (microplate well) equipment.
- In the future, it is necessary to validate the mixing performance of the micromixing devices under different conditions (materials, geometries, instrumentation setup). Additional research is also required to determine factors affecting the systematic dimensional errors found in certain components of the micromixing device.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AIDS | Acquired Immune Deficiency Syndrome |
| ASAR | Asymmetric split and recombination |
| CAD | Computer aided design |
| COC | Cyclic olefin copolymer |
| COP | Cyclic olefin polymer |
| DXF | Drawing Interchange Format |
| EP | Electrophoresis |
| FDM | Fused deposition modeling |
| SAR | Split and recombine |
| SGM | Slanted grooved mixer |
| SHM | Staggered herringbone mixer |
| PC | Polycarbonate |
| PET | Polyethylene terephthalate |
| PETG | Polyethylene terephthalate glycol |
| PDMS | Polydimethylsiloxane |
| PEEK | Polyether ether ketone |
| PMMA | Polymethyl methacrylate |
| POC | Point-of-Care |
| PVC | Polyvinyl chloride |
| RGB | Red-green-blue |
| SL | Stereolitography |
| TB | Tuberculosis |
| WHO | World Health Organization |
References
- Bissonnette, L.; Bergeron, M.G. Diagnosing infections—Current and anticipated technologies for point-of-care diagnostics and home-based testing. Clin. Microbiol. Infect. 2010, 16, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Mabey, D.; Peeling, R.W.; Ustianowski, A.; Perkins, M.D. Diagnostics for the developing world. Nat. Rev. Microbiol. 2004, 2, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Linder, V.; Sia, S.K. Lab-on-a-chip devices for global health: Past studies and future opportunities. Lab Chip 2006, 7, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Peeling, R.W. Evaluation of rapid diagnostic tests: Malaria. Nat. Rev. Microbiol. 2006, 4, S34–S38. [Google Scholar] [CrossRef] [PubMed]
- Banoo, S.; Bell, D.; Bossuyt, P.; Herring, A.; Mabey, D.; Poole, F.; Smith, P.G.; Sriram, N.; Wongsrichanalai, C.; Linke, R.; et al. Evaluation of diagnostic tests for infectious diseases: general principles. Nat. Rev. Microbiol. 2008, 8, S16–S28. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Chang-Yen, D.A.; Gale, B.K. Large-area, high-aspect-ratio SU-8 molds for the fabrication of PDMS microfluidic devices. J. Micromech. Microeng. 2008, 18, 045021. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Mogi, K.; Sugii, Y.; Yamamoto, T.; Fujii, T. Rapid fabrication technique of nano/microfluidic device with high mechanical stability utilizing two-step soft lithography. Sens. Actuators B Chem. 2014, 201, 407–412. [Google Scholar] [CrossRef]
- Martínez-López, J.I.; Mojica, M.; Rodríguez, C.A.; Siller, H.R. Xurography as a rapid fabrication alternative for point-of-care devices: Assessment of passive micromixers. Sensors 2016, 16, 705. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Medical applications for 3D printing: current and projected uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Yazdi, A.A.; Popma, A.; Wong, W.; Nguyen, T.; Pan, Y.; Xu, J. 3D printing: An emerging tool for novel microfluidics and lab-on-a-chip applications. Microfluid. Nanofluid. 2016, 20, 50. [Google Scholar] [CrossRef]
- Vig, A.L.; Mäkelä, T.; Majander, P.; Lambertini, V.; Ahopelto, J.; Kristensen, A. Roll-to-roll fabricated lab-on-a-chip devices. J. Micromech. Microeng. 2011, 21, 035006. [Google Scholar] [CrossRef]
- Deng, Y.; Yi, P.; Peng, L.; Lai, X.; Lin, Z. Flow behavior of polymers during the roll-to-roll hot embossing process. J. Micromech. Microeng. 2015, 25, 065004. [Google Scholar] [CrossRef]
- Liedert, R.; Amundsen, L.K.; Hokkanen, A.; Mäki, M.; Aittakorpi, A.; Pakanen, M.; Scherer, J.R.; Mathies, R.A.; Kurkinen, M.; Uusitalo, S.; et al. Disposable roll-to-roll hot embossed electrophoresis chip for detection of antibiotic resistance gene mecA in bacteria. Lab Chip 2011, 12, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeusz, D.A.; Boutte, R.W. Xurography: Rapid prototyping of microstructures using a cutting plotter. Microelectromech. Syst. J. 2006, 14, 1364–1374. [Google Scholar] [CrossRef]
- Treise, I.; Fortner, N.; Shapiro, B.; Hightower, A. Efficient energy based modeling and experimental validation of liquid filling in planar micro-fluidic components and networks. Lab Chip 2005, 5, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Silhouette America. Available online: https://www.silhouetteamerica.com/ (accessed on 14 April 2017).
- Cricut. Available online: https://home.cricut.com/ (accessed on 14 April 2017).
- Huang, H.; Guo, Z. Ultra-short pulsed laser PDMS thin-layer separation and micro-fabrication. J. Micromech. Microeng. 2009, 19, 055007. [Google Scholar] [CrossRef]
- Liao, Y.; Ju, Y.; Zhang, L.; He, F.; Zhang, Q.; Shen, Y.; Chen, D.; Cheng, Y.; Xu, Z.; Sugioka, K. Three-dimensional microfluidic channel with arbitrary length and configuration fabricated inside glass by femtosecond laser direct writing. Opt. Lett. 2010, 35, 3225–3227. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yakar, A.; Byer, R.L.; Harkin, A.; Ashmore, J.; Stone, H.A.; Shen, M.; Mazur, E. Morphology of femtosecond-laser-ablated borosilicate glass surfaces. Appl. Phys. Lett. 2003, 83, 3030–3032. [Google Scholar] [CrossRef]
- McCann, R.; Bagga, K.; Groarke, R.; Stalcup, A.; Vázquez, M.; Brabazon, D. Microchannel fabrication on cyclic olefin polymer substrates via 1064 nm Nd:YAG laser ablation. Appl. Surf. Sci. 2016, 387, 603–608. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Haag, R.; Bowden, N.; Whitesides, G.M. Generation of micrometer-sized patterns for microanalytical applications using a laser direct-write method and microcontact printing. Anal. Chem. 1998, 70, 4645–4652. [Google Scholar] [CrossRef]
- Hong, T.-F.; Ju, W.-J.; Wu, M.-C.; Tai, C.-H.; Tsai, C.-H.; Fu, L.-M. Rapid prototyping of PMMA microfluidic chips utilizing a CO2 laser. Microfluid. Nanofluid. 2010, 9, 1125–1133. [Google Scholar] [CrossRef]
- Malek, C.G.K. Laser processing for bio-microfluidics applications (part II). Anal. Bioanal. Chem. 2006, 385, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Pinecone Robotics. Available online: http://www.p-robots.com (accessed on 14 April 2017).
- Mr Beam-Laser Cutting for Everybody. Available online: http://mr-beam.org/#tech (accessed on 14 April 2017).
- Nguyen, N.-T.; Wu, Z. Micromixers—A review. J. Micromech. Microeng. 2005, 15, R1–R16. [Google Scholar] [CrossRef]
- Su, Y.; Chen, G.; Yuan, Q. Ideal micromixing performance in packed microchannels. Chem. Eng. Sci. 2011, 66, 2912–2919. [Google Scholar] [CrossRef]
- Schönfeld, F.; Hardt, S. Simulation of helical flows in microchannels. AIChE J. 2004, 50, 771–778. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Z.; Yim, C.; Lin, M.; Cao, X. Evaluation of floor-grooved micromixers using concentration-channel length profiles. Micromachines 2010, 1, 19–33. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.K.; Shih, T.R. Effect of geometry on fluid mixing of the rhombic micromixers. Microfluid. Nanofluid. 2007, 4, 419–425. [Google Scholar] [CrossRef]
- Hossain, S.; Kim, K.-Y. Mixing analysis of passive micromixer with unbalanced three-split rhombic sub-channels. Micromachines 2014, 5, 913–928. [Google Scholar] [CrossRef]
- Hong, C.-C.; Choi, J.-W.; Ahn, C.H. A novel in-plane passive micromixer using coanda effect. In Micro Total Analysis Systems 2001; Ramsey, J.M., van den Berg, A., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 31–33. [Google Scholar]
- Hong, C.-C.; Choi, J.-W.; Ahn, C.H. A novel in-plane passive microfluidic mixer with modified Tesla structures. Lab Chip 2004, 4, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Ansari, M.A.; Husain, A.; Kim, K.-Y. Analysis and optimization of a micromixer with a modified Tesla structure. Chem. Eng. J. 2010, 158, 305–314. [Google Scholar] [CrossRef]
- Sudarsan, A.P.; Ugaz, V.M. Fluid mixing in planar spiral microchannels. Lab Chip 2006, 6, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Kim, K.-Y.; Anwar, K.; Kim, S.M. A novel passive micromixer based on unbalanced splits and collisions of fluid streams. J. Micromech. Microeng. 2010, 20, 055007. [Google Scholar] [CrossRef]
- Ansari, M.A.; Kim, K.-Y. Mixing performance of unbalanced split and recombine micomixers with circular and rhombic sub-channels. Chem. Eng. J. 2010, 162, 760–767. [Google Scholar] [CrossRef]
- Chung, C.K.; Shih, T.R.; Wu, B.H.; Chang, C.K. Design and mixing efficiency of rhombic micromixer with flat angles. Microsyst. Technol. 2009, 16, 1595–1600. [Google Scholar] [CrossRef]
- Scherr, T.; Quitadamo, C.; Tesvich, P.; Park, D.S.-W.; Tiersch, T.; Hayes, D.; Choi, J.-W.; Nandakumar, K.; Monroe, W.T. A planar microfluidic mixer based on logarithmic spirals. J. Micromech. Microeng. 2012, 22, 055019. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, G.; Li, Y. Numerical and experimental analyses of planar asymmetric split-and-recombine micromixer with dislocation sub-channels. J. Chem. Technol. Biotechnol. 2013, 88, 1757–1765. [Google Scholar] [CrossRef]
- Martínez-López, J.I.; Mojica, M.; Betancourt, H.A.; Rodríguez, C.A.; Siller, H.R. Xurography and Lamination for Manufacturing Point-of-Care (POC) Micromixers. In Proceedings of the 4M/IWMF2016 Conference, Lyngby, Denmark, 13–15 September 2016; Research Publishing Services: Kgs. Lyngby, Denmark, 2016; pp. 227–230. [Google Scholar]
- Pai, N.P.; Vadnais, C.; Denkinger, C.; Engel, N.; Pai, M. Point-of-care testing for infectious diseases: Diversity, complexity, and barriers in low-and middle-income countries. PLoS Med. 2012, 9, e1001306. [Google Scholar] [CrossRef] [PubMed]
- Engel, N.; Ganesh, G.; Patil, M.; Yellappa, V.; Pai, N.P.; Vadnais, C.; Pai, M. Barriers to point-of-care testing in India: Results from qualitative research across different settings, users and major diseases. PLoS ONE 2015, 10, e0135112. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, J.I. Biosensing Enhancement of a SPR Imaging System Through Micromixing Structures; Tecnologico de Monterrey: Monterrey, Nuevo León, Mexico, 2013. [Google Scholar]
- Lynn, N.S.; Martínez-López, J.-I.; Bocková, M.; Adam, P.; Coello, V.; Siller, H.R.; Homola, J. Biosensing enhancement using passive mixing structures for microarray-based sensors. Biosens. Bioelectron. 2014, 54, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-J.; Maeng, J.-H.; Ahn, Y.; Hwang, S.Y. DNA ligation using a disposable microfluidic device combined with a micromixer and microchannel reactor. Sens. Actuators B Chem. 2011, 157, 735–741. [Google Scholar] [CrossRef]
- Danckwerts, P.V. The definition and measurement of some characteristics of mixtures. Appl. Sci. Res. Sect. A 1952, 3, 279–296. [Google Scholar]
- AForge.NET:: Computer Vision, Artificial Intelligence, Robotics. Available online: http://www.aforgenet.com/ (accessed on 28 February 2017).
- Langard, S.; Rosenberg, J.; Andersen, A.; Heldaas, S. Incidence of cancer among workers exposed to vinyl chloride in polyvinyl chloride manufacture. Occup. Environ. Med. 2000, 57, 65–68. [Google Scholar] [CrossRef] [PubMed]
- López, J.I.M.; Cervantes, H.A.B.; Garcia-Lopez, E.; Rodriguez, C.; Siller, H.R. Micromixing Test of a Four Channel Split and Recombine Array. Available online: https://doi.org/10.6084/m9.figshare.4704769.v1 (accessed on 26 April 2017).




| Work | Reference | Manufacture Methodology | N | winput |
|---|---|---|---|---|
| Hong et al. (2004) | [37] | Molding (nickel-SU-8), photolitography, hot embossing, drilling, thermal bonding | 1 | 200 μm |
| Sudarsan & Ugaz (2006) | [39] | Circuit printing, etching, heat treatment | 1 | 150 μm |
| Chung & Shi (2007) | [34] | Lithography, micro-molding, oxygen plasma treatment bonding, mechanical punching | 1 | 500 μm |
| Chung et al. (2009) | [42] | Laser machining, PDMS casting from PMMA, thermal and oxygen plasma bonding, mechanical punching | 1 | 500 μm |
| Ansari et al. (2010) | [40] | SU-8 photolithography over a silicon wafer, PDMS molding, mechanical punching | 1 | 300 μm |
| Scherr et al. (2012) | [43] | SU-8 photolithography, PDMS molding, plasma cleaning, mechanical punching | 1 | 30–200 μm |
| Li et al. (2013) | [44] | PDMS molding | 1 | 300 μm |
| Martínez-López et al. (2016) | [10] | Xurography of PVC and manual lamination | 1 | 750 μm |
| Setup | Manufacture Technology | Patterning Mechanism | Patterning Conditions | Testing Material |
|---|---|---|---|---|
| GX,OX,BX | Xurography: Graphtec CE5000-60 | Blade CB09U (45°) | Fload ≈ 0.8 N, Number of passes = 1 | Gray, Orange, Black 4500 CalPlus |
| GL,OL,FL | Laser ablation: Telesis EV25DS | Q-switched Nd: YVO4 laser | Mark speed = 500 mm/min, Frequency = 10 kHz, Laser power = 22.5 W, Pass number = 10 | Gray, Orange, Black 4500 CalPlus |
| Condition | Specification |
|---|---|
| Laser type | Class 4, fiber-coupled, diode-pumped, Q-switched Nd: YVO4 |
| Wavelength | 1064 nm |
| Mode | TEM_00 |
| Cooling system | Air-cooled |
| Galvanometer repeatibility | <22 micro radian |
| Field resolution | 16 bit (65,535 data points) |
| Marking field size (420 mm lens) | 290 × 290 mm |
| Mixing Array Element | Average Flow Velocity (U) | Reynolds Number (Re) | Mixing Efficiency (M) |
|---|---|---|---|
| I | 0.7 mm/s | 0.13 | 43.32% |
| II | 0.5 mm/s | 0.09 | 49.34% |
| III | 0.47 mm/s | 0.08 | 49.34% |
| IV | 0.38 mm/s | 0.07 | 65.08% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-López, J.I.; Betancourt, H.A.; García-López, E.; Rodriguez, C.A.; Siller, H.R. Rapid Fabrication of Disposable Micromixing Arrays Using Xurography and Laser Ablation. Micromachines 2017, 8, 144. https://doi.org/10.3390/mi8050144
Martínez-López JI, Betancourt HA, García-López E, Rodriguez CA, Siller HR. Rapid Fabrication of Disposable Micromixing Arrays Using Xurography and Laser Ablation. Micromachines. 2017; 8(5):144. https://doi.org/10.3390/mi8050144
Chicago/Turabian StyleMartínez-López, J. Israel, H.A. Betancourt, Erika García-López, Ciro A. Rodriguez, and Hector R. Siller. 2017. "Rapid Fabrication of Disposable Micromixing Arrays Using Xurography and Laser Ablation" Micromachines 8, no. 5: 144. https://doi.org/10.3390/mi8050144
APA StyleMartínez-López, J. I., Betancourt, H. A., García-López, E., Rodriguez, C. A., & Siller, H. R. (2017). Rapid Fabrication of Disposable Micromixing Arrays Using Xurography and Laser Ablation. Micromachines, 8(5), 144. https://doi.org/10.3390/mi8050144