The Dilemma of Cure and Damage in Oligodendroglioma: Ways to Tip the Balance Away from the Damage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cancer Stem Cells Are the Cycling Cells in Oligodendroglioma and the Targets of RTC
2.2. Diminishing the Damaging Effects by Lowering the Dose at the Start of the Chemotherapeutics
2.3. The MRI Monitors the Size of the Tumor
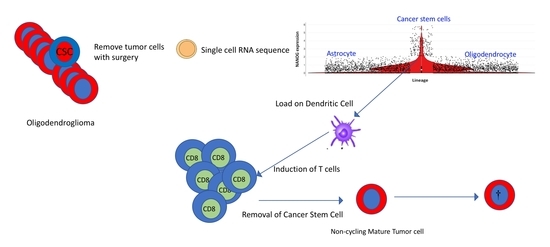
2.4. Prospects for Optimal Tumor Removal by Surgery and Immunotherapy
2.5. Dendritic Cell (DC) Vaccination with Stem Cell Antigen
3. Materials and Methods
Patient Materials
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Piai, V.; Vos, S.H.; Idelberger, R.; Gans, P.; Doorduin, J.; Ter Laan, M. Awake Surgery for a Violin Player: Monitoring Motor and Music Performance, A Case Report. Arch. Clin. Neuropsychol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pirro, V.; Alfaro, C.M.; Jarmusch, A.K.; Hattab, E.M.; Cohen-Gadol, A.A.; Cooks, R.G. Intraoperative assessment of tumor margins during glioma resection by desorption electrospray ionization-mass spectrometry. Proc. Natl. Acad Sci. USA 2017, 114, 6700–6705. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. Surgical oncology for gliomas: The state of the art. Nat. Rev. Clin. Oncol. 2017, 15, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.C.; Shaw, E.G.; Pugh, S.L.; Chakravarti, A.; Gilbert, M.R.; Barger, G.R.; Coons, S.; Ricci, P.; Bullard, D.; Brown, P.D.; et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N. Engl. J. Med. 2016, 374, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Cairncross, G.; Wang, M.; Shaw, E.; Jenkins, R.; Brachman, D.; Buckner, J.; Fink, K.; Souhami, L.; Laperriere, N.; Curran, W.; et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: Long-term results of RTOG 9402. J. Clin. Oncol. 2013, 31, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Van den Bent, M.J.; Brandes, A.A.; Taphoorn, M.J.; Kros, J.M.; Kouwenhoven, M.C.; Delattre, J.Y.; Bernsen, H.J.; Frenay, M.; Tijssen, C.C.; Grisold, W.; et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013, 31, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Cairncross, J.G.; Wang, M.; Jenkins, R.B.; Shaw, E.G.; Giannini, C.; Brachman, D.G.; Buckner, J.C.; Fink, K.L.; Souhami, L.; Laperriere, N.J.; et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J. Clin. Oncol. 2014, 32, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.G.; Wang, M.; Coons, S.W.; Brachman, D.G.; Buckner, J.C.; Stelzer, K.J.; Barger, G.R.; Brown, P.D.; Gilbert, M.R.; Mehta, M.P. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: Initial results of RTOG 9802. J. Clin. Oncol. 2012, 30, 3065–3070. [Google Scholar] [CrossRef] [PubMed]
- Kaloshi, G.; Roci, E.; Rroji, A.; Ducray, F.; Petrela, M. Kinetic evaluation of low-grade gliomas in adults before and after treatment with CCNU alone. J. Neurosurg. 2015, 123, 1244–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirosh, I.; Venteicher, A.S.; Hebert, C.; Escalante, L.E.; Patel, A.P.; Yizhak, K.; Fisher, J.M.; Rodman, C.; Mount, C.; Filbin, M.G.; et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature 2016, 539, 309–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J. Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacol. Ther. 2016, 160, 145–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wefers, C.; Schreibelt, G.; Massuger, L.F.; de Vries, J.M.; Torensma, R. Immune curbing of cancer stem cells by CTLs directed to NANOG. Front. Immunol. 2018, 9, 1412. [Google Scholar] [CrossRef] [PubMed]
- Venteicher, A.S.; Tirosh, I.; Hebert, C.; Yizhak, K.; Neftel, C.; Filbin, M.G.; Hovestadt, V.; Escalante, L.E.; Shaw, M.L.; Rodman, C.; et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 2017, 355, eaai8478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Bent, M.J.; Kros, J.M.; Heimans, J.J.; Pronk, L.C.; van Groeningen, C.J.; Krouwer, H.G.; Taphoorn, M.J.; Zonnenberg, B.A.; Tijssen, C.C.; Twijnstra, A.; et al. Response rate and prognostic factors of recurrent oligodendroglioma treated with procarbazine, CCNU, and vincristine chemotherapy. Dutch Neuro-oncology Group. Neurology 1998, 51, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.D.; Gilbert, M.R. Clinical discussion of the management of anaplastic oligodendroglioma/oligoastrocytoma (both codeleted and nondeleted). J. Natl. Compr. Cancer Netw. 2014, 12, 665–672. [Google Scholar] [CrossRef]
- Bhowmik, A.; Khan, R.; Ghosh, M.K. Blood brain barrier: A challenge for effectual therapy of brain tumors. Biomed. Res. Int. 2015, 2015, 320941. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, I.; Suva, M.L. Dissecting human gliomas by single-cell RNA sequencing. Neuro Oncol. 2018, 20, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bradford, G.B.; Williams, B.; Rossi, R.; Bertoncello, I. Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp. Hematol. 1997, 25, 445–453. [Google Scholar] [PubMed]
- Liu, R.; Page, M.; Solheim, K.; Fox, S.; Chang, S.M. Quality of life in adults with brain tumors: Current knowledge and future directions. Neuro Oncol. 2009, 11, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrey, L.E.; Louis, D.N.; Paleologos, N.; Lassman, A.B.; Raizer, J.J.; Mason, W.; Finlay, J.; MacDonald, D.R.; DeAngelis, L.M.; Cairncross, J.G.; et al. Survey of treatment recommendations for anaplastic oligodendroglioma. Neuro Oncol. 2007, 9, 314–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassman, A.B.; Iwamoto, F.M.; Cloughesy, T.F.; Aldape, K.D.; Rivera, A.L.; Eichler, A.F.; Louis, D.N.; Paleologos, N.A.; Fisher, B.J.; Ashby, L.S.; et al. International retrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol. 2011, 13, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speirs, C.K.; Simpson, J.R.; Robinson, C.G.; DeWees, T.A.; Tran, D.D.; Linette, G.; Chicoine, M.R.; Dacey, R.G.; Rich, K.M.; Dowling, J.L.; et al. Impact of 1p/19q codeletion and histology on outcomes of anaplastic gliomas treated with radiation therapy and temozolomide. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Starobova, H.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Prestwood, T.R.; van der Linden, W.A.; Carmi, Y.; Bhattacharya, N.; Withana, N.; Verdoes, M.; Habtezion, A.; Engleman, E.G.; Bogyo, M. Detection of Intestinal Cancer by Local, Topical Application of a Quenched Fluorescence Probe for Cysteine Cathepsins. Chem. Biol. 2015, 22, 148–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butte, P.V.; Mamelak, A.; Parrish-Novak, J.; Drazin, D.; Shweikeh, F.; Gangalum, P.R.; Chesnokova, A.; Ljubimova, J.Y.; Black, K. Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg. Focus 2014, 36, E1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, D.W. Applications of fluorescent technology in neurosurgery. Neurosurg. Focus 2014, 36, E2. [Google Scholar] [CrossRef] [PubMed]
- Pogue, B.W.; Zhu, T.C.; Ntziachristos, V.; Paulsen, K.D.; Wilson, B.C.; Pfefer, J.; Nordstrom, R.J.; Litorja, M.; Wabnitz, H.; Chen, Y.; et al. Fluorescence-guided surgery and intervention—An AAPM emerging technology blue paper. Med. Phys. 2018, 45, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santagata, S.; Eberlin, L.S.; Norton, I.; Calligaris, D.; Feldman, D.R.; Ide, J.L.; Liu, X.; Wiley, J.S.; Vestal, M.L.; Ramkissoon, S.H.; et al. Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery. Proc. Natl. Acad. Sci. USA 2014, 111, 11121–11126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sans, M.; Feider, C.L.; Eberlin, L.S. Advances in mass spectrometry imaging coupled to ion mobility spectrometry for enhanced imaging of biological tissues. Curr. Opin. Chem. Biol. 2018, 42, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Pirro, V.; Llor, R.S.; Jarmusch, A.K.; Alfaro, C.M.; Cohen-Gadol, A.A.; Hattab, E.M.; Cooks, R.G. Analysis of human gliomas by swab touch spray-mass spectrometry: Applications to intraoperative assessment of surgical margins and presence of oncometabolites. Analyst 2017, 142, 4058–4066. [Google Scholar] [CrossRef] [PubMed]
- Sans, M.; Gharpure, K.; Tibshirani, R.; Zhang, J.; Liang, L.; Liu, J.; Young, J.H.; Dood, R.L.; Sood, A.K.; Eberlin, L.S. Metabolic Markers and Statistical Prediction of Serous Ovarian Cancer Aggressiveness by Ambient Ionization Mass Spectrometry Imaging. Cancer Res. 2017, 77, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.M.; Pirro, V.; Cooks, R.G. From DESI to the MasSpec Pen: Ambient Ionization Mass Spectrometry for Tissue Analysis and Intrasurgical Cancer Diagnosis. Clin. Chem. 2018, 64, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Ferracci, F.-X.; Duffau, H. Improving surgical outcome for gliomas with intraoperative mapping. Expert Rev. Neurother. 2018, 18, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Van Ark, T.J.; Klimek, M.; de Smalen, P.; Vincent, A.; Stolker, R.J. Anxiety, memories and coping in patients undergoing intracranial tumor surgery. Clin. Neurol. Neurosurg. 2018, 170, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Zacharakis, N.; Chinnasamy, H.; Black, M.; Xu, H.; Lu, Y.-C.; Zheng, Z.; Pasetto, A.; Langhan, M.; Shelton, T.; Prickett, T.; et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 2018, 24, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Antonios, J.P.; Everson, R.G.; Liau, L.M. Dendritic cell immunotherapy for brain tumors. J. Neurooncol. 2015, 123, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Pellegatta, S. Immunotherapy with dendritic cells loaded with glioblastoma stem cells: From preclinical to clinical studies. Cancer Immunol. Immunother. 2016, 65, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.; Grauer, O.M.; Kamp, M.; Sevens, N.; Zotz, N.; Sabel, M.; Sorg, R.V. A randomized controlled phase II trial of vaccination with lysate-loaded, mature dendritic cells integrated into standard radiochemotherapy of newly diagnosed glioblastoma (GlioVax): Study protocol for a randomized controlled trial. Trials 2018, 19, 293. [Google Scholar] [CrossRef] [PubMed]
- Pellegatta, S.; Eoli, M.; Cuccarini, V.; Anghileri, E.; Pollo, B.; Pessina, S.; Frigerio, S.; Servida, M.; Cuppini, L.; Antozzi, C.; et al. Survival gain in glioblastoma patients treated with dendritic cell immunotherapy is associated with increased NK but not CD8(+) T cell activation in the presence of adjuvant temozolomide. Oncoimmunology 2018, 7, e1412901. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Trenevska, I.; Li, D.; Banham, A.H. Therapeutic Antibodies against Intracellular Tumor Antigens. Front. Immunol. 2017, 8, 1001. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.; Bunse, L.; Pusch, S.; Sahm, F.; Wiestler, B.; Quandt, J.; Menn, O.; Osswald, M.; Oezen, I.; Ott, M.; et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 2014, 512, 324. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, B.; Tobias, A.L.; Han, Y.; Lee, G.; Guo, D.; Dey, M.; Lesniak, M.S.; Ahmed, A.U. Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ. 2014, 21, 1119–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmez, I.; Shen, W.; McDonald, H.; Ozpolat, B. Dedifferentiation of patient-derived glioblastoma multiforme cell lines results in a cancer stem cell-like state with mitogen-independent growth. J. Cell. Mol. Med. 2015, 19, 1262–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torensma, R. The Dilemma of Cure and Damage in Oligodendroglioma: Ways to Tip the Balance Away from the Damage. Cancers 2018, 10, 431. https://doi.org/10.3390/cancers10110431
Torensma R. The Dilemma of Cure and Damage in Oligodendroglioma: Ways to Tip the Balance Away from the Damage. Cancers. 2018; 10(11):431. https://doi.org/10.3390/cancers10110431
Chicago/Turabian StyleTorensma, Ruurd. 2018. "The Dilemma of Cure and Damage in Oligodendroglioma: Ways to Tip the Balance Away from the Damage" Cancers 10, no. 11: 431. https://doi.org/10.3390/cancers10110431
APA StyleTorensma, R. (2018). The Dilemma of Cure and Damage in Oligodendroglioma: Ways to Tip the Balance Away from the Damage. Cancers, 10(11), 431. https://doi.org/10.3390/cancers10110431