Novel Thienopyrimidine Derivative, RP-010, Induces β-Catenin Fragmentation and Is Efficacious against Prostate Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. RP-010 has Efficacy Against PC Cell Lines
2.2. RP-010 Blocks the PC Cell Cycle at the G2 Phase
2.3. RP-010 Increases Oxidative Stress in PC Cells
2.4. RP-010 Kills DU145 and PC-3 PC Cells by Mitotic Catastrophe and Apoptosis
2.5. RP-010 Induces Apoptosis by the Pro-Apoptotic Bcl-2 Family Proteins, Inducing Caspase Activation
2.6. RP-010 Inhibits PC Cell Migration
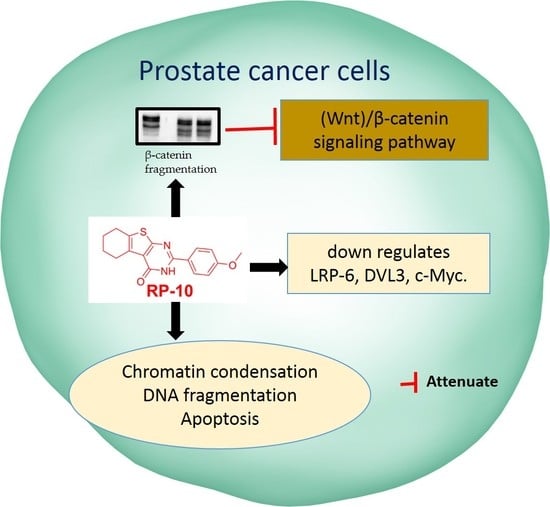
2.7. RP-010 Downregulates Wnt/β-Catenin Signaling Pathway Proteins in PC Cells, In Vitro
2.8. Effect of RP-010 in an In Vivo Zebrafish Model of Toxicity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines and Culture
4.3. Cell Cytotoxicity Assays
4.3.1. MTT Assay
4.3.2. Colony Formation Assay
4.3.3. Time-Dependent Studies with RP-010
4.4. Cell Cycle Analysis
4.5. Detection of Oxidative Stress in PC Cells
4.6. DAPI Staining
4.7. Migration Assays
4.7.1. Wound Healing Assays
4.7.2. Transwell Migration Assays
4.8. Subcellular Fractionation and Western Analysis
4.9. Evaluation of RP-010 Toxicity, in Zebrafish, In Vivo
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Miller, K.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Selley, S.; Donovan, J.; Faulkner, A.; Coast, J.; Gillatt, D. Diagnosis, management and screening of early localised prostate cancer. Health Technol Assess. 1997, 1, 1–96. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.-J. The diagnosis and treatment of prostate cancer: A review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Carroll, P.R. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA 2015, 314, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Ahlering, T.; Skarecky, D. Long-term outcome of detectable PSA levels after radical prostatectomy. Prostate Cancer Prostatic Dis. 2005, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceder, J.; Elgqvist, J. Targeting prostate cancer stem cells with alpha-particle therapy. Front. Oncol. 2017, 6, 273. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.B.; Dits, N.F.; Erkens-Schulze, S.; van Weerden, W.M.; Jenster, G. Bypass mechanisms of the androgen receptor pathway in therapy-resistant prostate cancer cell models. PLoS ONE 2010, 5, e13500. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Polakis, P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008052. [Google Scholar] [CrossRef]
- White, B.D.; Chien, A.J.; Dawson, D.W. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology 2012, 142, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, N.N.; Shao, S.; Hoang, B.H.; Mercola, D.; Zi, X. Wnt signaling in castration-resistant prostate cancer: Implications for therapy. Am. J. Clin. Exp. Urol. 2014, 2, 27. [Google Scholar]
- Ghith, A.; Ismail, N.S.; Youssef, K.; Abouzid, K.A. Medicinal Attributes of Thienopyrimidine Based Scaffold Targeting Tyrosine Kinases and Their Potential Anticancer Activities. Arch. Der Pharm. 2017, 350, 1700242. [Google Scholar] [CrossRef]
- Amawi, H.; Karthikeyan, C.; Pathak, R.; Hussein, N.; Christman, R.; Robey, R.; Ashby, C.R., Jr.; Trivedi, P.; Malhotra, A.; Tiwari, A.K. Thienopyrimidine derivatives exert their anticancer efficacy via apoptosis induction, oxidative stress and mitotic catastrophe. Eur. J. Med. Chem. 2017, 138, 1053–1065. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Banerjee, S.; Kong, D.; Ahmad, A.; Nogueira, V.; Hay, N.; Sarkar, F.H. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J. Cell Biochem. 2010, 109, 726–736. [Google Scholar] [CrossRef]
- Wakeling, A.E.; Guy, S.P.; Woodburn, J.R.; Ashton, S.E.; Curry, B.J.; Barker, A.J.; Gibson, K.H. ZD1839 (Iressa): An orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002, 62, 5749–5754. [Google Scholar]
- DeAngelo, D.J.; Stone, R.M.; Heaney, M.L.; Nimer, S.D.; Paquette, R.L.; Klisovic, R.B.; Caligiuri, M.A.; Cooper, M.R.; Lecerf, J.-M.; Karol, M.D. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: Safety, pharmacokinetics, and pharmacodynamics. Blood 2006, 108, 3674–3681. [Google Scholar] [CrossRef]
- Gangjee, A.; Qiu, Y.; Kisliuk, R.L. Synthesis of classical and nonclassical 2-amino-4-oxo-6-benzylthieno-[2, 3-d] pyrimidines as potential thymidylate synthase inhibitors. J. Heterocycl. Chem. 2004, 41, 941–946. [Google Scholar] [CrossRef]
- Shaaban, M.A.; Ghorab, M.M.; Heiba, H.I.; Kamel, M.M.; Zaher, N.H.; Mostafa, M.I. Novel Thiophenes, Thienopyrimidines, and Triazolothienopyrimidines for the Evaluation of Anticancer and Augmentation Effects of γ-Radiation. Arch. Pharm. 2010, 343, 404–410. [Google Scholar] [CrossRef]
- Salib, S.B.; Khalil, O.M.; Kamel, M.M.; El-Dash, Y. Synthesis and antitumor activity of novel thienopyrimidine derivatives containing thiosemicarbazide moiety. Open Access Libr. J. 2016, 3, 1. [Google Scholar] [CrossRef]
- Fujino, M.; Fukuda, T.; Shinagawa, S.; Kobayashi, S.; Yamazaki, I.; Nakayama, R.; Seely, J.; White, W.; Rippel, R. Synthetic analogs of luteinizing hormone releasing hormone (LH-RH) substituted in position 6 and 10. Biochem. Biophys. Res. Commun. 1974, 60, 406–413. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Wu, D.; Zhu, Y.-F.; Struthers, R.S.; Saunders, J.; Xie, Q.; Chen, C. Synthesis and structure–Activity relationships of thieno [2, 3-d] pyrimidine-2, 4-dione derivatives as potent GnRH receptor antagonists. Bioorg. Med. Chem. Lett. 2003, 13, 3617–3622. [Google Scholar] [CrossRef]
- Johnston, J.B.; Navaratnam, S.; Pitz, M.W.; Maniate, J.M.; Wiechec, E.; Baust, H.; Gingerich, J.; Skliris, G.P.; Murphy, L.C.; Los, M. Targeting the EGFR pathway for cancer therapy. Curr. Med. Chem. 2006, 13, 3483–3492. [Google Scholar] [CrossRef] [PubMed]
- Rheault, T.R.; Caferro, T.R.; Dickerson, S.H.; Donaldson, K.H.; Gaul, M.D.; Goetz, A.S.; Mullin, R.J.; McDonald, O.B.; Petrov, K.G.; Rusnak, D.W. Thienopyrimidine-based dual EGFR/ErbB-2 inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 817–820. [Google Scholar] [CrossRef]
- Andreassen, P.R.; Lacroix, F.B.; Lohez, O.D.; Margolis, R.L. Neither p21WAF1 nor 14-3-3σ prevents G2 progression to mitotic catastrophe in human colon carcinoma cells after DNA damage, but p21WAF1 induces stable G1 arrest in resulting tetraploid cells. Cancer Res. 2001, 61, 7660–7668. [Google Scholar] [PubMed]
- Castedo, M.; Perfettini, J.-L.; Roumier, T.; Andreau, K.; Medema, R.; Kroemer, G. Cell death by mitotic catastrophe: A molecular definition. Oncogene 2004, 23, 2825. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; LINskENs, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- D’Alise, A.M.; Amabile, G.; Iovino, M.; Di Giorgio, F.P.; Bartiromo, M.; Sessa, F.; Villa, F.; Musacchio, A.; Cortese, R. Reversine, a novel Aurora kinases inhibitor, inhibits colony formation of human acute myeloid leukemia cells. Mol. Cancer Ther. 2008, 7, 1140–1149. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.-C.; Lee, Y.-R.; Liao, J.-D.; Lin, C.-Y.; Chen, Y.-Y.; Chen, P.-T.; Tseng, Y.-S. Reversine induced multinucleated cells, cell apoptosis and autophagy in human non-small cell lung cancer cells. PLoS ONE 2016, 11, e0158587. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Lu, Y.-C.; Tseng, Y.-S.; Shi, C.-S.; Chen, S.-H.; Chen, P.-T.; Wu, F.-L.; Chang, Y.-P.; Lee, Y.-R. Reversine induces cell cycle arrest, polyploidy, and apoptosis in human breast cancer cells. Breast Cancer 2014, 21, 358–369. [Google Scholar] [CrossRef]
- Eom, Y.-W.; Kim, M.A.; Park, S.S.; Goo, M.J.; Kwon, H.J.; Sohn, S.; Kim, W.-H.; Yoon, G.; Choi, K.S. Two distinct modes of cell death induced by doxorubicin: Apoptosis and cell death through mitotic catastrophe accompanied by senescence-like phenotype. Oncogene 2005, 24, 4765. [Google Scholar] [CrossRef]
- Chen, C.-A.; Chen, C.-C.; Shen, C.-C.; Chang, H.-H.; Chen, Y.-J. Moscatilin induces apoptosis and mitotic catastrophe in human esophageal cancer cells. J. Med. Food 2013, 16, 869–877. [Google Scholar] [CrossRef]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef]
- Danial, N.N. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin. Cancer Res. 2007, 13, 7254–7263. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr. Opin. Immunol. 2007, 19, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Stancikova, J.; Krausova, M.; Kolar, M.; Fafilek, B.; Svec, J.; Sedlacek, R.; Neroldova, M.; Dobes, J.; Horazna, M.; Janeckova, L. NKD1 marks intestinal and liver tumors linked to aberrant Wnt signaling. Cell. Signal. 2015, 27, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of β-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef]
- Sprowl, S.; Waterman, M.L. Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity. PLoS Genet. 2013, 9, e1003745. [Google Scholar] [CrossRef] [PubMed]
- Chiurillo, M.A. Role of the Wnt/β-catenin pathway in gastric cancer: An in-depth literature review. World J. Exp. Med. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; He, X.; Jia, M.; Liu, Y.; Qu, D.; Wu, D.; Wu, P.; Ni, C.; Zhang, Z.; Ye, J. β-catenin overexpression in the nucleus predicts progress disease and unfavourable survival in colorectal cancer: A meta-analysis. PLoS ONE 2013, 8, e63854. [Google Scholar] [CrossRef]
- Francis, J.C.; Thomsen, M.K.; Taketo, M.M.; Swain, A. β-catenin is required for prostate development and cooperates with Pten loss to drive invasive carcinoma. PLoS Genet. 2013, 9, e1003180. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, Y.; Jiang, M.; Bierie, B.; Roy-Burman, P.; Shen, M.M.; Taketo, M.M.; Wills, M.; Matusik, R.J. Activation of β-Catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. Prostate 2009, 69, 249–262. [Google Scholar] [CrossRef]
- Chesire, D.R.; Ewing, C.M.; Gage, W.R.; Isaacs, W.B. In vitro evidence for complex modes of nuclear β-catenin signaling during prostate growth and tumorigenesis. Oncogene 2002, 21, 2679. [Google Scholar] [CrossRef]
- De la Taille, A.; Rubin, M.A.; Chen, M.-W.; Vacherot, F.; de Medina, S.G.-D.; Burchardt, M.; Buttyan, R.; Chopin, D. β-Catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin. Cancer Res. 2003, 9, 1801–1807. [Google Scholar]
- Wan, X.; Liu, J.; Lu, J.-F.; Tzelepi, V.; Yang, J.; Starbuck, M.W.; Diao, L.; Wang, J.; Efstathiou, E.; Vazquez, E.S. Activation of β-Catenin Signaling in Androgen Receptor–Negative Prostate Cancer Cells. Clin. Cancer Res. 2012, 18, 726–736. [Google Scholar] [CrossRef]
- Bisson, I.; Prowse, D.M. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009, 19, 683. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Rai, N.; Jana, K.; Ghatak, S.; Basu, A.; Mustafi, S.B.; Raha, S. Beta catenin is degraded by both caspase-3 and proteasomal activity during resveratrol-induced apoptosis in HeLa cells in a GSK3β-independent manner. Indian J. Biochem. Biophys. 2015, 52, 7–13. [Google Scholar]
- Amawi, H.; Hussein, N.A.; Karthikeyan, C.; Manivannan, E.; Wisner, A.; Williams, F.E.; Samuel, T.; Trivedi, P.; Ashby, C.R., Jr.; Tiwari, A.K. HM015k, a novel silybin derivative, multi-targets metastatic ovarian cancer cells and is safe in zebrafish toxicity studies. Front. Pharmacol. 2017, 8, 498. [Google Scholar] [CrossRef]
- Amawi, H.; Hussein, N.A.; Ashby, C.R., Jr.; Alnafisah, R.; Sanglard, L.M.; ELANGOVAN, M.; Chandrabose, K.; Trivedi, P.; Eisenmann, K.M.; Robey, R.R. Bax/tubulin/epithelial-mesenchymal pathways determine the efficacy of silybin analog HM015k in colorectal cancer cell growth and metastasis. Front. Pharmacol. 2018, 9, 520. [Google Scholar] [CrossRef]
- Özhan, G.; Sezgin, E.; Wehner, D.; Pfister, A.S.; Kühl, S.J.; Kagermeier-Schenk, B.; Kühl, M.; Schwille, P.; Weidinger, G. Lypd6 enhances Wnt/β-catenin signaling by promoting Lrp6 phosphorylation in raft plasma membrane domains. Dev. Cell 2013, 26, 331–345. [Google Scholar] [CrossRef]
- Orian-Rousseau, V.; Schmitt, M. CD44 regulates Wnt signaling at the level of LRP6. Mol. Cell. Oncol. 2015, 2, e995046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, B.; Giri, B.; Majumder, K.; Dudeja, V.; Banerjee, S.; Saluja, A. Modulation of post-translational modifications in β-catenin and LRP6 inhibits Wnt signaling pathway in pancreatic cancer. Cancer Lett. 2017, 388, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.J.; Park, J.Y.; Kim, H.G.; Cho, Y.M.; Go, H. Dishevelled segment polarity protein 3 (DVL3): A novel and easily applicable recurrence predictor in localised prostate adenocarcinoma. BJU Int. 2017, 120, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Bajrami, I.; Verrill, C.; Kigozi, A.; Ouaret, D.; Aleksic, T.; Asher, R.; Han, C.; Allen, P.; Bailey, D. Dsh homolog DVL3 mediates resistance to IGFIR inhibition by regulating IGF-RAS signaling. Cancer Res. 2014, 74, 5866–5877. [Google Scholar] [CrossRef] [PubMed]
- Conacci-Sorrell, M.; Zhurinsky, J.; Ben-Ze’ev, A. The cadherin-catenin adhesion system in signaling and cancer. J. Clin. Investig. 2002, 109, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Kurenbekova, L.; Gao, Y.; Roos, A.; Creighton, C.; Rao, P.; Hicks, J.; Man, T.; Lau, C.; Brown, A. NKD2, a negative regulator of Wnt signaling, suppresses tumor growth and metastasis in osteosarcoma. Oncogene 2015, 34, 5069. [Google Scholar] [CrossRef]
- Dong, Y.; Cao, B.; Zhang, M.; Han, W.; Herman, J.G.; Fuks, F.; Zhao, Y.; Guo, M. Epigenetic silencing of NKD2, a major component of Wnt signaling, promotes breast cancer growth. Oncotarget 2015, 6, 22126. [Google Scholar] [CrossRef]
- Hu, T.; Li, C.; Cao, Z.; Van Raay, T.J.; Smith, J.G.; Willert, K.; Solnica-Krezel, L.; Coffey, R.J. Myristoylated Naked2 antagonizes Wnt-β-catenin activity by degrading Dishevelled-1 at the plasma membrane. J. Biol. Chem. 2010, 285, 13561–13568. [Google Scholar] [CrossRef]
- Van Raay, T.J.; Fortino, N.J.; Miller, B.W.; Ma, H.; Lau, G.; Li, C.; Franklin, J.L.; Attisano, L.; Solnica-Krezel, L.; Coffey, R.J. Naked1 antagonizes Wnt signaling by preventing nuclear accumulation of β-catenin. PLoS ONE 2011, 6, e18650. [Google Scholar] [CrossRef]
- Jia, Y.; Cao, B.; Yang, Y.; Linghu, E.; Zhan, Q.; Lu, Y.; Yu, Y.; Herman, J.G.; Guo, M. Silencing NKD2 by promoter region hypermethylation promotes gastric cancer invasion and metastasis by up-regulating SOX18 in human gastric cancer. Oncotarget 2015, 6, 33470. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.W.; Sohn, J.H.; Kim, D.-H.; Choi, Y.J.; Park, Y.L.; Kim, K.; Cho, Y.H.; Pyo, J.-S.; Kim, J.H. Overexpressions of Cyclin B1, cdc2, p16 and p53 in human breast cancer: The clinicopathologic correlations and prognostic implications. Yonsei Med J. 2011, 52, 445–453. [Google Scholar] [CrossRef]
- Aaltonen, K.; Amini, R.-M.; Heikkilä, P.; Aittomäki, K.; Tamminen, A.; Nevanlinna, H.; Blomqvist, C. High cyclin B1 expression is associated with poor survival in breast cancer. Br. J. Cancer 2009, 100, 1055. [Google Scholar] [CrossRef]
- Han, J.-H.; Lee, J.; Jeon, S.-J.; Choi, E.-S.; Cho, S.-D.; Kim, B.-Y.; Kim, D.-J.; Park, J.-H.; Park, J.-H. In vitro and in vivo growth inhibition of prostate cancer by the small molecule imiquimod. Int. J. Oncol. 2013, 42, 2087–2093. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Liang, X.; Jiang, W.; Li, J.; Xu, J.; Cai, X. Cyclin b1 suppresses colorectal cancer invasion and metastasis by regulating e-cadherin. PLoS ONE 2015, 10, e0126875. [Google Scholar] [CrossRef]
- Androic, I.; Krämer, A.; Yan, R.; Rödel, F.; Gätje, R.; Kaufmann, M.; Strebhardt, K.; Yuan, J. Targeting cyclin B1 inhibits proliferation and sensitizes breast cancer cells to taxol. BMC Cancer 2008, 8, 391. [Google Scholar] [CrossRef]
- Calvisi, D.F.; Conner, E.A.; Ladu, S.; Lemmer, E.R.; Factor, V.M.; Thorgeirsson, S.S. Activation of the canonical Wnt/β-catenin pathway confers growth advantages in c-Myc/E2F1 transgenic mouse model of liver cancer. J. Hepatol. 2005, 42, 842–849. [Google Scholar] [CrossRef]
- Brabletz, T.; Herrmann, K.; Jung, A.; Faller, G.; Kirchner, T. Expression of nuclear β-catenin and c-myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am. J. Pathol. 2000, 156, 865–870. [Google Scholar] [CrossRef]
- Heerboth, S. Housman g, leary m, longacre m. Byler S, lapinska K, Willbanks a and Sarkar S: Emt and tumor metastasis. Clin. Transl. Med. 2015, 4, 6. [Google Scholar] [CrossRef]
- Arias, A.M. Epithelial mesenchymal interactions in cancer and development. Cell 2001, 105, 425–431. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R.; Samuel, T.; Peraman, R.; Tiwari, A.K. Polyphenolic nutrients in cancer chemoprevention and metastasis: Role of the epithelial-to-mesenchymal (EMT) pathway. Nutrients 2017, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Rajasekaran, A.K. Dishonorable discharge: The oncogenic roles of cleaved E-cadherin fragments. Cancer Res. 2012, 72, 2917–2923. [Google Scholar] [CrossRef]
- Huber, M.A.; Kraut, N.; Beug, H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 2005, 17, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, E.; Amawi, H.; Hussein, N.; Karthikeyan, C.; Fetcenko, A.; Moorthy, N.H.N.; Trivedi, P.; Tiwari, A.K. Design and discovery of silybin analogues as antiproliferative compounds using a ring disjunctive–Based, natural product lead optimization approach. Eur. J. Med. Chem. 2017, 133, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Amawi, H.; Viana, A.G.; Sanglard, L.; Hussein, N.; Saddler, M.; Ashby, C.R., Jr.; Moorthy, N.; Trivedi, P.; Tiwari, A.K. lH-Pyrazolo[3,4-b]quinolin-3-amine derivatives inhibit growth of colon cancer cells via apoptosis and sub G1 cell cycle arrest. Bioorg. Med. Chem. Lett. 2018, 28, 2244–2249. [Google Scholar] [CrossRef]
- Albini, A.; Dell’Eva, R.; Vene, R.; Ferrari, N.; Buhler, D.R.; Noonan, D.M.; Fassina, G. Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NF-kappaB and Akt as targets. FASEB J. 2006, 20, 527–529. [Google Scholar] [CrossRef]






| RP Series | IC50 ± SD (µM) | |||
|---|---|---|---|---|
| Prostate Cancer Cells | Non-Prostate Cells | |||
| PC-3 | DU145 | CRL-1459 | CHO | |
| RP1 | >100 | >100 | >100 | >100 |
| RP2 | >100 | >100 | >100 | >100 |
| RP3 | >100 | >100 | >100 | >100 |
| RP4 | 73.4 ± 3.1 | >100 | >100 | >100 |
| RP5 | >100 | >100 | >100 | >100 |
| RP6 | >100 | >100 | >100 | >100 |
| RP7 | >100 | >100 | >100 | >100 |
| RP8 | 6.4 ± 0.5 | >100 | >100 | >100 |
| RP9 | 2.8 ± 1.1 | >100 | >100 | >100 |
| RP10 | 0.3 ± 0.1 | 0.5 ± 0.2 | 73.0 ± 5.3 | 14.0 ± 1.3 |
| RP11 | 6.6 ± 0.7 | 2.7 ± 0.5 | >100 | >100 |
| RP12 | 6.9 ± 1.0 | >100 | >100 | >100 |
| RP13 | >100 | >100 | >100 | >100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amawi, H.; Hussein, N.; Boddu, S.H.S.; Karthikeyan, C.; Williams, F.E.; Ashby, C.R., Jr.; Raman, D.; Trivedi, P.; Tiwari, A.K. Novel Thienopyrimidine Derivative, RP-010, Induces β-Catenin Fragmentation and Is Efficacious against Prostate Cancer Cells. Cancers 2019, 11, 711. https://doi.org/10.3390/cancers11050711
Amawi H, Hussein N, Boddu SHS, Karthikeyan C, Williams FE, Ashby CR Jr., Raman D, Trivedi P, Tiwari AK. Novel Thienopyrimidine Derivative, RP-010, Induces β-Catenin Fragmentation and Is Efficacious against Prostate Cancer Cells. Cancers. 2019; 11(5):711. https://doi.org/10.3390/cancers11050711
Chicago/Turabian StyleAmawi, Haneen, Noor Hussein, Sai H. S. Boddu, Chandrabose Karthikeyan, Frederick E. Williams, Charles R. Ashby, Jr., Dayanidhi Raman, Piyush Trivedi, and Amit K. Tiwari. 2019. "Novel Thienopyrimidine Derivative, RP-010, Induces β-Catenin Fragmentation and Is Efficacious against Prostate Cancer Cells" Cancers 11, no. 5: 711. https://doi.org/10.3390/cancers11050711
APA StyleAmawi, H., Hussein, N., Boddu, S. H. S., Karthikeyan, C., Williams, F. E., Ashby, C. R., Jr., Raman, D., Trivedi, P., & Tiwari, A. K. (2019). Novel Thienopyrimidine Derivative, RP-010, Induces β-Catenin Fragmentation and Is Efficacious against Prostate Cancer Cells. Cancers, 11(5), 711. https://doi.org/10.3390/cancers11050711