In Vivo Assessment of VCAM-1 Expression by SPECT/CT Imaging in Mice Models of Human Triple Negative Breast Cancer
Abstract
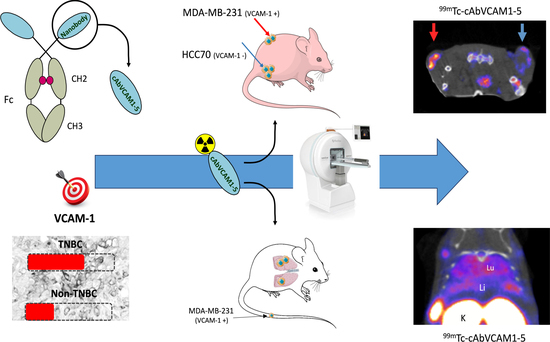
:1. Introduction
2. Results
2.1. VCAM-1 Is Overexpressed in TNBC and Associated with Decreased OS
2.2. MDA-MB-231 Cells Overexpress VCAM-1 mRNA and Protein
2.3. 99mTc-cAbVCAM1-5 Binds Specifically to VCAM-1 Expressing TNBC Cells
2.4. SPECT/CT Imaging of VCAM-1 in Subcutaneous Tumor Model
2.5. Ex Vivo Assessment of VCAM-1 Expression in Subcutaneous Tumors
2.6. SPECT/CT Imaging of VCAM-1 in an Experimental Metastasis Model
2.7. Ex Vivo Assessment of VCAM-1 Expression in the Experimental Metastasis Model
3. Discussion
4. Materials and Methods
4.1. Patients—Online Data
4.2. Cell Lines and Culture Conditions
4.3. RT-qPCR Assay
4.4. Western Blot Assay
4.5. Flow Cytometry
4.6. Saturation Binding Experiments and In Vitro Competition Studies
4.7. Tumor Models
4.8. SPECT/CT Imaging
4.9. Post-Mortem Analysis
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perou, C.M.; Sorlie, T.B.; Eisen, C.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis-Filho, J.S.; Tutt, A.N.J. Triple negative tumours: A critical review: Triple negative tumours. Histopathology 2007, 52, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haffty, B.G.; Yang, Q.; Reiss, M.; Kearney, T.; Higgins, S.A.; Weidhaas, J.; Harris, L.; Hait, W.; Toppmeyer, D. Locoregional Relapse and Distant Metastasis in Conservatively Managed Triple Negative Early-Stage Breast Cancer. J. Clin. Oncol. 2006, 24, 5652–5657. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, G.; Rüegg, C. New insights into the mechanisms of organ-specific breast cancer metastasis. Semin. Cancer Biol. 2012, 22, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Minn, A.J.; Gupta, G.P.; Siegel, P.M.; Bos, P.D.; Shu, W.; Giri, D.D.; Viale, A.; Olshen, A.B.; Gerald, W.L.; Massagué, J. Genes that mediate breast cancer metastasis to lung. Nature 2005, 436, 518. [Google Scholar] [CrossRef]
- Chen, Q.; Massague, J. Molecular Pathways: VCAM-1 as a Potential Therapeutic Target in Metastasis. Clin. Cancer Res. 2012, 18, 5520–5525. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011, 15, 1607–1638. [Google Scholar] [CrossRef]
- Schlesinger, M.; Bendas, G. Vascular cell adhesion molecule-1 (VCAM-1)-An increasing insight into its role in tumorigenicity and metastasis: VCAM-1 in tumorigenicity. Int. J. Cancer 2015, 136, 2504–2514. [Google Scholar] [CrossRef] [PubMed]
- Maimaiti, Y.; Wang, C.; Mushajiang, M.; Tan, J.; Huang, B.; Zhou, J.; Huang, T. Overexpression of VCAM-1 is correlated with poor survival of patients with breast cancer. Int. J. Clin. Exp. Pathol. 2016, 9, 7451–7457. [Google Scholar]
- Lu, X.; Mu, E.; Wei, Y.; Riethdorf, S.; Yang, Q.; Yuan, M.; Yan, J.; Hua, Y.; Tiede, B.J.; Lu, X.; et al. VCAM-1 Promotes Osteolytic Expansion of Indolent Bone Micrometastasis of Breast Cancer by Engaging α4β1-Positive Osteoclast Progenitors. Cancer Cell 2011, 20, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, X.H.-F.; Massagué, J. Macrophage Binding to Receptor VCAM-1 Transmits Survival Signals in Breast Cancer Cells that Invade the Lungs. Cancer Cell 2011, 20, 538–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y. Dissecting Tumor-Stromal Interactions in Breast Cancer Bone Metastasis. Endocrinol. Metab. 2016, 31, 206. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhang, Z.; Zhao, S.; He, X.; Yu, H.; Yin, Q.; Zhang, Z.; Gu, W.; Chen, L.; Li, Y. Hydrophobic interaction mediating self-assembled nanoparticles of succinobucol suppress lung metastasis of breast cancer by inhibition of VCAM-1 expression. J. Control. Release 2015, 205, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-C. The Role of Vascular Cell Adhesion Molecule-1 in Tumor Immune Evasion. Cancer Res. 2007, 67, 6003–6006. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-Y.; Lu, D.; Hung, C.-F.; Peng, S.; Huang, L.; Jie, C.; Murillo, F.; Rowley, J.; Tsai, Y.-C.; He, L.; et al. Ectopic Expression of Vascular Cell Adhesion Molecule-1 as a New Mechanism for Tumor Immune Evasion. Cancer Res. 2007, 67, 1832–1841. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.-C.; Weng, C.-C.; Hou, Y.-S.; Jian, S.-F.; Fang, K.-T.; Hou, M.-F.; Cheng, K.-H. Activation of VCAM-1 and Its Associated Molecule CD44 Leads to Increased Malignant Potential of Breast Cancer Cells. Int. J. Mol. Sci. 2014, 15, 3560–3579. [Google Scholar] [CrossRef] [Green Version]
- Broisat, A.; Hernot, S.; Toczek, J.; De Vos, J.; Riou, L.M.; Martin, S.; Ahmadi, M.; Thielens, N.; Wernery, U.; Caveliers, V.; et al. Nanobodies Targeting Mouse/Human VCAM1 for the Nuclear Imaging of Atherosclerotic Lesions. Circ. Res. 2012, 30, 927–937. [Google Scholar] [CrossRef]
- Dumas, L.S.; Briand, F.; Clerc, R.; Brousseau, E.; Montemagno, C.; Ahmadi, M.; Bacot, S.; Soubies, A.; Perret, P.; Riou, L.M.; et al. Evaluation of Antiatherogenic Properties of Ezetimibe Using 3H-Labeled Low-Density-Lipoprotein Cholesterol and 99mTc-cAbVCAM1–5 SPECT in ApoE−/− Mice Fed the Paigen Diet. J. Nucl. Med. 2017, 58, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, A.B.; Etzioni, R.; Hurlbert, M.; Penberthy, L.; Mayer, M. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol. Biomark. Prev. 2017, 26, 809–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Group EBCTC. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Eckhardt, B.L.; Francis, P.A.; Parker, B.S.; Anderson, R.L. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat. Rev. Drug Discov. 2012, 11, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, R.; Khaket, T.P.; Dutta, C.; Chakraborty, B.; Mukherjee, T.K. Breast cancer metastasis: Putative therapeutic role of vascular cell adhesion molecule-1. Cell Oncol. 2017, 40, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-B.; Chen, G.-Y.; Xia, J.-G.; Zang, X.-W.; Yang, H.-Y.; Yang, L. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J. Gastroenterol. 2003, 9, 1409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-S.; Lin, H.-Y.; Lai, S.-W.; Huang, C.-Y.; Huang, B.-R.; Chen, P.-Y.; Wei, K.-C.; Lu, D.-Y. MiR-181b modulates EGFR-dependent VCAM-1 expression and monocyte adhesion in glioblastoma. Oncogene 2017, 36, 5006–5022. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J.; Li, H.; Lu, Z.; Shan, W.; Mercado-Uribe, I.; Liu, J. VCAM1 expression correlated with tumorigenesis and poor prognosis in high grade serous ovarian cancer. Am. J. Transl. Res. 2013, 5, 336. [Google Scholar]
- Zhang, X.; Liu, C.; Hu, F.; Zhang, Y.; Wang, J.; Gao, Y.; Jiang, Y.; Zhang, Y.; Lan, X. PET Imaging of VCAM-1 Expression and Monitoring Therapy Response in Tumor with a 68Ga-Labeled Single Chain Variable Fragment. Mol. Pharm. 2018, 15, 609–618. [Google Scholar] [CrossRef]
- Scalici, J.M.; Thomas, S.; Harrer, C.; Raines, T.A.; Curran, J.; Atkins, K.A.; Conaway, M.R.; Duska, L.; Kelly, K.A.; Slack-Davis, J.K. Imaging VCAM-1 as an Indicator of Treatment Efficacy in Metastatic Ovarian Cancer. J. Nucl. Med. 2013, 54, 1883–1889. [Google Scholar] [CrossRef] [Green Version]
- Broisat, A.; Toczek, J.; Dumas, L.S.; Ahmadi, M.; Bacot, S.; Perret, P.; Slimani, L.; Barone-Rochette, G.; Soubies, A.; Devoogdt, N.; et al. 99mTc-cAbVCAM1-5 Imaging Is a Sensitive and Reproducible Tool for the Detection of Inflamed Atherosclerotic Lesions in Mice. J. Nucl. Med. 2014, 55, 1678–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, S.; Ye, J.; Bai, S.; Huang, F.; Guo, Y.-L. NF-κB, but not p38 MAP Kinase, is required for TNF-α-induced expression of cell adhesion molecules in endothelial cells. J. Cell. Biochem. 2008, 105, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Vincent, A.; Cates, J.; Brantley-Sieders, D.M.; Polk, D.B.; Young, P.P. Low Levels of Tumor Necrosis Factor Increase Tumor Growth by Inducing an Endothelial Phenotype of Monocytes Recruited to the Tumor Site. Cancer Res. 2009, 69, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Cao, C.; Li, J.; Chen, F.; Zhang, S.; Liu, B.; Zhang, W.; Zhang, X.; Ye, L. Inflammatory factor TNF-alpha promotes the growth of breast cancer via the positive feedback loop of TNFR1/NF-κB (and/or p38)/p-STAT3/HBXIP/TNFR1. Oncotarget 2017, 8, 58338–58352. [Google Scholar] [PubMed]
- The cBioPortal for Cancer Genomics. Available online: https://www.cbioportal.org/study/summary?id=brca_tcga (accessed on 19 July 2019).
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data: Figure 1. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montemagno, C.; Dumas, L.; Cavaillès, P.; Ahmadi, M.; Bacot, S.; Debiossat, M.; Soubies, A.; Djaïleb, L.; Leenhardt, J.; De Leiris, N.; et al. In Vivo Assessment of VCAM-1 Expression by SPECT/CT Imaging in Mice Models of Human Triple Negative Breast Cancer. Cancers 2019, 11, 1039. https://doi.org/10.3390/cancers11071039
Montemagno C, Dumas L, Cavaillès P, Ahmadi M, Bacot S, Debiossat M, Soubies A, Djaïleb L, Leenhardt J, De Leiris N, et al. In Vivo Assessment of VCAM-1 Expression by SPECT/CT Imaging in Mice Models of Human Triple Negative Breast Cancer. Cancers. 2019; 11(7):1039. https://doi.org/10.3390/cancers11071039
Chicago/Turabian StyleMontemagno, Christopher, Laurent Dumas, Pierre Cavaillès, Mitra Ahmadi, Sandrine Bacot, Marlène Debiossat, Audrey Soubies, Loic Djaïleb, Julien Leenhardt, Nicolas De Leiris, and et al. 2019. "In Vivo Assessment of VCAM-1 Expression by SPECT/CT Imaging in Mice Models of Human Triple Negative Breast Cancer" Cancers 11, no. 7: 1039. https://doi.org/10.3390/cancers11071039
APA StyleMontemagno, C., Dumas, L., Cavaillès, P., Ahmadi, M., Bacot, S., Debiossat, M., Soubies, A., Djaïleb, L., Leenhardt, J., De Leiris, N., Dufies, M., Pagès, G., Hernot, S., Devoogdt, N., Perret, P., Riou, L., Fagret, D., Ghezzi, C., & Broisat, A. (2019). In Vivo Assessment of VCAM-1 Expression by SPECT/CT Imaging in Mice Models of Human Triple Negative Breast Cancer. Cancers, 11(7), 1039. https://doi.org/10.3390/cancers11071039