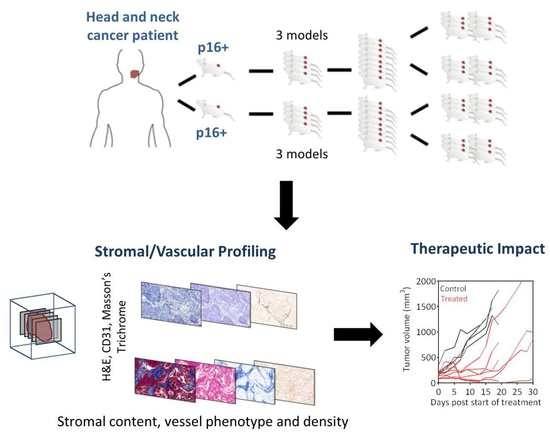
Profiling the Stromal and Vascular Heterogeneity in Patient-derived Xenograft Models of Head and Neck Cancer: Impact on Therapeutic Response
Abstract
:1. Introduction
2. Results
2.1. Establishing a Panel of PDX Models of HNSCC
2.2. Profiling the Stromal and Vascular Heterogeneity in PDX Models of HNSCC.
2.3. Validation of Vascular Phenotypes in Human HNSCC
2.4. Early Pathologic Response and Therapeutic Efficacy of VDA Therapy in PDX Models of HNSCC
2.5. Impact of Phenotype on the Susceptibility of Vessels to VDA Therapy
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Procurement of Human Tumor Tissue and Establishing of Xenografts
4.3. Immunostaining of PDX
4.4. Image Segmentation and Morphometric Analysis
4.5. Human HNSCC Tissue Microarray
4.6. Drug Treatment
4.7. Therapeutic Response Assessment
4.8. Magnetic Resonance Imaging
4.9. Sample Sizes and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patel, S.G.; Shah, J.P. TNM staging of cancers of the head and neck: Striving for uniformity among diversity. CA Cancer J. Clin. 2005, 55, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Mroz, E.A.; Tward, A.D.; Pickering, C.R.; Myers, J.N.; Ferris, R.L.; Rocco, J.W. High intratumor genetic heterogeneity is related to worse outcome in patients with head and neck squamous cell carcinoma. Cancer 2013, 119, 3034–3042. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Chiosea, S.I.; Grandis, J.R. Molecular changes in the multistage pathogenesis of head and neck cancer. Cancer Biomark. 2010, 9, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Cognetti, D.M.; Weber, R.S.; Lai, S.Y. Head and neck cancer: An evolving treatment paradigm. Cancer 2008, 113, 1911–1932. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, S.; Hidalgo, M.; Kung, A.L. Examining the utility of patient-derived xenograft mouse models. Nat. Rev. Cancer 2015, 15, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Gengenbacher, N.; Singhal, M.; Augustin, H.G. Preclinical mouse solid tumour models: Status quo, challenges and perspectives. Nat. Rev. Cancer 2017, 17, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.T.; Alférez, D.G.; Amant, F.; Annibali, D.; Arribas, J.; Biankin, A.V.; Bruna, A.; Budinská, E.; Caldas, C.; Chang, D.K.; et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 2017, 17, 254–268. [Google Scholar] [CrossRef]
- Seshadri, M.; Merzianu, M.; Tang, H.; Rigual, N.R.; Sullivan, M.; Loree, T.R.; Popat, S.R.; Repasky, E.A.; Hylander, B.L. Establishment and characterization of patient tumor-derived head and neck squamous cell carcinoma xenografts. Cancer Biol. Ther. 2009, 8, 2275–2283. [Google Scholar] [CrossRef]
- Kimple, R.J.; Harari, P.M.; Torres, A.D.; Yang, R.Z.; Soriano, B.J.; Yu, M.; Armstrong, E.A.; Blitzer, G.C.; Smith, M.A.; Lorenz, L.D.; et al. Development and characterization of HPV-positive and HPV-negative head and neck squamous cell carcinoma tumor grafts. Clin. Cancer Res. 2013, 19, 855–864. [Google Scholar] [CrossRef]
- Peng, S.; Creighton, C.J.; Zhang, Y.; Sen, B.; Mazumdar, T.; Myers, J.N.; Lai, S.Y.; Woolfson, A.; Lorenzi, M.V.; Bell, D.; et al. Tumor grafts derived from patients with head and neck squamous carcinoma authentically maintain the molecular and histologic characteristics of human cancers. J. Transl. Med. 2013, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Gourdeau, H.; Leblond, L.; Hamelin, B.; Desputeau, C.; Dong, K.; Kianicka, I.; Custeau, D.; Boudreau, C.; Geerts, L.; Cai, S.X.; et al. Antivascular and antitumor evaluation of 2-amino-4-(3-bromo-4,5-dimethoxyphenyl)-3-cyano-4H-chromenes, a novel series of anticancer agents. Mol. Cancer Ther. 2004, 3, 1375–1384. [Google Scholar] [PubMed]
- Cai, S.X.; Drewe, J.; Kemnitzer, W. Discovery of 4-aryl-4H-chromenes as potent apoptosis inducers using a cell- and caspase-based Anti-cancer Screening Apoptosis Program (ASAP): SAR studies and the identification of novel vascular disrupting agents. Anticancer Agents Med. Chem. 2009, 9, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Kalmuk, J.; Folaron, M.; Buchinger, J.; Pili, R.; Seshadri, M. Multimodal imaging guided preclinical trials of vascular targeting in prostate cancer. Oncotarget 2015, 6, 24376–24392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folaron, M.; Seshadri, M. Bioluminescence and MR Imaging of the Safety and Efficacy of Vascular Disruption in Gliomas. Mol. Imaging Biol. 2016, 18, 860–869. [Google Scholar] [CrossRef]
- Munger, K.; Baldwin, A.; Edwards, K.M.; Hayakawa, H.; Nguyen, C.L.; Owens, M.; Grace, M.; Huh, K. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004, 78, 11451–11460. [Google Scholar] [CrossRef]
- Zheng, Z.; Baker, C.C. Papillomavirus genome structure, expression dna post-transcriptional regulation. Front. Biosci. 2006, 11, 2286–2302. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Smith, N.R.; Baker, D.; Farren, M.; Pommier, A.; Swann, R.; Wang, X.; Mistry, S.; McDaid, K.; Kendrew, J.; Womack, C.; et al. Tumor stromal architecture can define the intrinsic tumor response to VEGF-targeted therapy. Clin. Cancer Res. 2013, 19, 6943–6956. [Google Scholar] [CrossRef]
- Subbiah, I.M.; Lenihan, D.J.; Tsimberidou, A.M. Cardiovascular toxicity profiles of vascular-disrupting agents. Oncologist 2011, 16, 1120–1130. [Google Scholar] [CrossRef]
- Hasina, R.; Whipple, M.E.; Martin, L.E.; Kuo, W.P.; Ohno-Machado, L.; Lingen, M.W. Angiogenic heterogeneity in head and neck squamous cell carcinoma: Biological and therapeutic implications. Lab. Investig. 2008, 88, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Rustum, Y.M.; Tóth, K.; Seshadri, M.; Sen, A.; Durrani, F.A.; Stott, E.; Morrison, C.D.; Cao, S.; Bhattacharya, A. Architectural heterogeneity in tumors caused by differentiation alters intratumoral drug distribution and affects therapeutic synergy of antiangiogenic organoselenium compound. J. Oncol. 2010, 2010, 396286. [Google Scholar] [CrossRef] [PubMed]
- Baruah, P.; Lee, M.; Wilson, P.O.; Odutoye, T.; Williamson, P.; Hyde, N.; Kaski, J.C.; Dumitriu, I.E. Impact of p16 status on pro- and anti-angiogenesis factors in head and neck cancers. Br. J. Cancer 2015, 113, 653–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troy, J.D.; Weissfeld, J.L.; Youk, A.O.; Thomas, S.; Wang, L.; Grandis, J.R. Expression of EGFR, VEGF, and NOTCH1 suggest differences in tumor angiogenesis in HPV-positive and HPV-negative head and neck squamous cell carcinoma. Head Neck Pathol. 2013, 7, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Hauff, S.J.; Raju, S.C.; Orosco, R.K.; Gross, A.M.; Diaz-Perez, J.A.; Savariar, E.; Nashi, N.; Hasselman, J.; Whitney, M.; Myers, J.N.; et al. Matrix-metalloproteinases in head and neck carcinoma-cancer genome atlas analysis and fluorescence imaging in mice. Otolaryngol. Head Neck Surg. 2014, 151, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.; Li, R.; Eisele, D.; Fakhry, C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014, 50, 565–574. [Google Scholar] [CrossRef]
- Fakhry, C.; Gillison, M.L. Clinical implications of human papillomavirus in head and neck cancers. J. Clin. Oncol. 2006, 24, 2606–2611. [Google Scholar] [CrossRef]
- Helfrich, I.; Scheffrahn, I.; Bartling, S.; Weis, J.; von Felbert, V.; Middleton, M.; Kato, M.; Ergün, S.; Augustin, H.G.; Schadendorf, D. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J. Exp. Med. 2010, 207, 491–503. [Google Scholar] [CrossRef] [Green Version]
- Lassen, P.; Eriksen, J.G.; Hamilton-Dutoit, S.; Tramm, T.; Alsner, J.; Overgaard, J.; Danish Head and Neck Cancer Group (DAHANCA). HPV-associated p16-expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiother. Oncol. 2010, 94, 30–35. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Boucher, Y.; Duda, D.G.; Martin, J.D.; Seano, G.; Ancukiewicz, M.; Barry, W.T.; Goel, S.; Lahdenrata, J.; Isakoff, S.J.; et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc. Natl. Acad. Sci. USA 2015, 112, 14325–14330. [Google Scholar] [CrossRef] [Green Version]
- Kamat, C.D.; Green, D.E.; Warnke, L.; Thorpe, J.E.; Ceriello, A.; Ihnat, M.A. Mutant p53 facilitates pro-angiogenic, hyperproliferative phenotype in response to chronic relative hypoxia. Cancer Lett. 2007, 249, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Said, R.; Hong, D.S.; Warneke, C.L.; Lee, J.J.; Wheler, J.J.; Janku, F.; Naing, A.; Falchook, G.S.; Fu, S.; Piha-Paul, S.; et al. P53 mutations in advanced cancers: Clinical characteristics, outcomes, and correlation between progression-free survival and bevacizumab-containing therapy. Oncotarget 2013, 4, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, M.; Mazurchuk, R.; Spernyak, J.A.; Bhattacharya, A.; Rustum, Y.M.; Bellnier, D.A. Activity of the vascular-disrupting agent 5,6-dimethylxanthenone-4-acetic acid against human head and neck carcinoma xenografts. Neoplasia 2006, 8, 534–542. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folaron, M.; Merzianu, M.; Duvvuri, U.; Ferris, R.L.; Seshadri, M. Profiling the Stromal and Vascular Heterogeneity in Patient-derived Xenograft Models of Head and Neck Cancer: Impact on Therapeutic Response. Cancers 2019, 11, 951. https://doi.org/10.3390/cancers11070951
Folaron M, Merzianu M, Duvvuri U, Ferris RL, Seshadri M. Profiling the Stromal and Vascular Heterogeneity in Patient-derived Xenograft Models of Head and Neck Cancer: Impact on Therapeutic Response. Cancers. 2019; 11(7):951. https://doi.org/10.3390/cancers11070951
Chicago/Turabian StyleFolaron, Margaret, Mihai Merzianu, Umamaheswar Duvvuri, Robert L. Ferris, and Mukund Seshadri. 2019. "Profiling the Stromal and Vascular Heterogeneity in Patient-derived Xenograft Models of Head and Neck Cancer: Impact on Therapeutic Response" Cancers 11, no. 7: 951. https://doi.org/10.3390/cancers11070951
APA StyleFolaron, M., Merzianu, M., Duvvuri, U., Ferris, R. L., & Seshadri, M. (2019). Profiling the Stromal and Vascular Heterogeneity in Patient-derived Xenograft Models of Head and Neck Cancer: Impact on Therapeutic Response. Cancers, 11(7), 951. https://doi.org/10.3390/cancers11070951