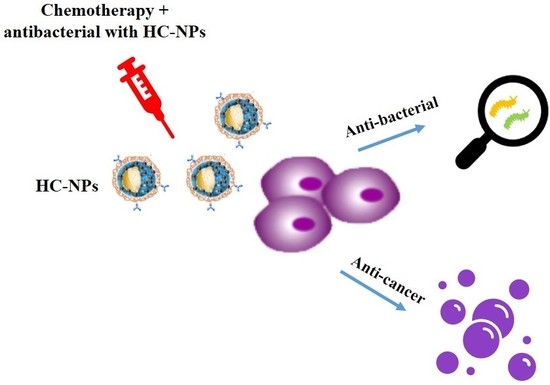
Hybrid Clustered Nanoparticles for Chemo-Antibacterial Combinatorial Cancer Therapy
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of Hybrid Cluster NPs
2.2. Protein Corona Analysis
2.3. Antibacterial Action
2.4. Targeting and Cellular Uptake Analysis
2.5. Anti-Cancer Efficacy of HC-NPs
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of AgNPs
4.2. Synthesis of HC-NPs
4.2.1. Synthesis of IM Loaded PCL NPs
4.2.2. Synthesis of HC-NPs
4.2.3. HC-NPs Functionalization with Anti-CD38 Antibody
4.3. Characterization of NPs
4.3.1. Dynamic Light Scattering
4.3.2. UV-VIS Spectroscopy
4.3.3. Scanning and Transmission Electron Microscopy
4.3.4. FT-IR Spectroscopy
4.3.5. Flow Cytometry and SDS-PAGE Assay
4.3.6. Protein Corona
4.3.7. Hemolytic Activity
4.4. Targeting and HC-NP Cellular Uptake
4.5. In Vitro Cancer Efficacy
4.5.1. Apoptosis and Cell Cycle Analysis
4.5.2. Western Blotting for BCR-ABL Inhibition Analysis
4.5.3. Superoxide Dismutase (SOD) Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yap, T.A.; Omlin, A.; Bono, J.S.D. Development of Therapeutic Combinations Targeting Major Cancer Signaling Pathways. J. Clin. Oncol. 2013, 31, 1592–1605. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Winer, E.P.; Burstein, H.J. Side Effects of Chemotherapy and Combined Chemohormonal Therapy in Women with Early-Stage Breast Cancer. JNCI Monogr. 2001, 2001, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Salvatorelli, E.; Minotti, G. Anthracycline cardiotoxicity in breast cancer patients: Synergism with trastuzumab and taxanes. Cardiovasc. Toxicol. 2007, 7, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.; Merry, S. Tumor-Cell Resistance to Anthracyclines—A Review. Cancer Chemother. Pharmacol. 1985, 14, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, A.; Tai, E.; Nielsen, D.B.; Shropshire, S.; Richardson, L.C. Preventing Infections During Cancer Treatment: Development of an Interactive Patient Education Website. Clin. J. Oncol. Nurs. 2014, 18, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Pala, N.; Sechi, M. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771. [Google Scholar] [CrossRef]
- Cortese, B.; D’Amone, S.; Palama, I.E. Wool-Like Hollow Polymeric Nanoparticles for CML Chemo-Combinatorial Therapy. Pharmaceutics 2018, 10, 52. [Google Scholar] [CrossRef]
- Nam, J.; La, W.-G.; Hwang, S.; Ha, Y.S.; Park, N.; Won, N.; Jung, S.; Bhang, S.H.; Ma, Y.-J.; Cho, Y.-M.; et al. pH-Responsive Assembly of Gold Nanoparticles and “Spatiotemporally Concerted” Drug Release for Synergistic Cancer Therapy. ACS Nano 2013, 7, 3388–3402. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Z.; Cao, D.; Zhao, Y.-L.; Gaines, J.W.; Bozdemir, O.A.; Ambrogio, M.W.; Frasconi, M.; Botros, Y.Y.; Zink, J.I.; et al. Stimulated Release of Size-Selected Cargos in Succession from Mesoporous Silica Nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 5460–5465. [Google Scholar] [CrossRef] [PubMed]
- Moorthi, C.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to Overcome Conventional Cancer Chemotherapy Limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef]
- Ostad, S.N.; Dehnad, S.; Nazari, Z.E.; Fini, S.T.; Mokhtari, N.; Shakibaie, M.; Shahverdi, A.R. Cytotoxic Activities of Silver Nanoparticles and Silver Ions in Parent and Tamoxifen-Resistant T47D Human Breast Cancer Cells and Their Combination Effects with Tamoxifen against Resistant Cells. Avicenna J. Med Biotechnol. 2010, 2, 187–196. [Google Scholar] [PubMed]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic Nanoparticles in Cancer Therapy. Pharm. Res. 2011, 28, 237–259. [Google Scholar] [CrossRef]
- Elbaz, N.M.; Ziko, L.; Siam, R.; Mamdouh, W. Core-Shell Silver/Polymeric Nanoparticles-Based Combinatorial Therapy against Breast Cancer In-vitro. Sci. Rep. 2016, 6, 30729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palama, I.E.; Pollini, M.; Paladini, F.; Accorsi, G.; Sannino, A.; Gigli, G.; Palama, I. Inhibiting Growth or Proliferation of a Cancer Cell e.g., Myeloid Leukemia Cell, Comprises Contacting the Cancer Cell with Silver Nanoparticles. WO2015015301-A2 WOIB001895, 31 July 2014. [Google Scholar]
- Guo, D.; Zhu, L.; Huang, Z.; Zhou, H.; Ge, Y.; Ma, W.; Wu, J.; Zhang, X.; Zhou, X.; Zhang, Y.; et al. Anti-leukemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions. Biomaterials 2013, 34, 7884–7894. [Google Scholar] [CrossRef]
- Heravi, R.E.; Zakeri, S.; Nazari, P. Anticancer activity evaluation of green synthesised gold-silver alloy nanoparticles on colourectal HT-29 and prostate DU-145 carcinoma cell lines. Micro Nano Lett. 2018, 13, 1475–1479. [Google Scholar] [CrossRef]
- De Matteis, V.; Malvindi, M.A.; Galeone, A.; Brunetti, V.; De Luca, E.; Kote, S.; Kshirsagar, P.; Sabella, S.; Bardi, G.; Pompa, P.P. Negligible particle-specific toxicity mechanism of silver nanoparticles: The role of Ag+ ion release in the cytosol. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 731–739. [Google Scholar] [CrossRef]
- Richa, S.; Dimple, S.C. Regulatory Approval of Silver Nanoparticles. Appl. Clin. Res. Clin. Trials Regul. Aff. 2018, 5, 74–79. [Google Scholar] [CrossRef]
- Vardiman, J.W. Chronic Myelogenous Leukemia, BCR-ABL1+. Am. J. Clin. Pathol. 2009, 132, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Yalcintepe, L.; Halis, E.; Ulku, S. Effect of CD38 on the multidrug resistance of human chronic myelogenous leukemia K562 cells to doxorubicin. Oncol. Lett. 2016, 11, 2290–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palamà, I.E.; Leporatti, S.; Luca, E.D.; Renzo, N.D.; Maffia, M.; Gambacorti-Passerini, C.; Rinaldi, R.; Gigli, G.; Cingolani, R.; Coluccia, A.M. Imatinib-loaded polyelectrolyte microcapsules for sustained targeting of BCR-ABL+ leukemia stem cells. Nanomedicine 2010, 5, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, A.A.; Li, X.L.; El-Nabarawi, M.; Elassasy, A.; Jasti, B. Effect of fixed aqueous layer thickness of polymeric stabilizers on zeta potential and stability of aripiprazole nanosuspensions. Pharm. Dev. Technol. 2013, 18, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Sadzuka, Y.; Nakade, A.; Hirama, R.; Miyagishima, A.; Nozawa, Y.; Hirota, S.; Sonobe, T. Effects of mixed polyethyleneglycol modification on fixed aqueous layer thickness and antitumor activity of doxorubicin containing liposome. Int. J. Pharm. 2002, 238, 171–180. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [PubMed]
- Murugan, C.; Rayappan, K.; Thangam, R.; Bhanumathi, R.; Shanthi, K.; Vivek, R.; Thirumurugan, R.; Bhattacharyya, A.; Sivasubramanian, S.; Gunasekaran, P.; et al. Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in breast cancer cells: An improved nanomedicine strategy. Sci. Rep. 2016, 6, 34053. [Google Scholar] [CrossRef] [PubMed]
- Palamà, I.E.; Coluccia, A.M.; Gigli, G. Uptake of imatinib-loaded polyelectrolyte complexes by BCR-ABL+ cells: A long-acting drug-delivery strategy for targeting oncoprotein activity. Nanomedicine 2014, 9, 2087–2098. [Google Scholar] [CrossRef]
- Cortese, B.; D’Amone, S.; Gigli, G.; Palama, I.E. Sustained anti-BCR-ABL activity with pH responsive imatinib mesylate loaded PCL nanoparticles in CML cells. MedChemComm 2015, 6, 212–221. [Google Scholar] [CrossRef]
- Gujrati, M.; Malamas, A.; Shin, T.; Jin, E.; Sun, Y.; Lu, Z.-R. Multifunctional Cationic Lipid-Based Nanoparticles Facilitate Endosomal Escape and Reduction-Triggered Cytosolic siRNA Release. Mol. Pharm. 2014, 11, 2734–2744. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Miceli, E.; Kar, M.; Calderón, M. Interactions of organic nanoparticles with proteins in physiological conditions. J. Mater. Chem. B 2017, 5, 4393–4405. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Taraballi, F.; Toledano Furman, N.E.; Hartman, K.A.; Sherman, M.B.; De Rosa, E.; Kirui, D.K.; Salvatore, F.; Tasciotti, E. Unveiling the in Vivo Protein Corona of Circulating Leukocyte-like Carriers. ACS Nano 2017, 11, 3262–3273. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Bagherifam, S.; Bekhradnia, S.; Nyström, B. Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line. Sci. Rep. 2017, 7, 5178. [Google Scholar] [CrossRef]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]







| Sample | ζ Potential (mV) | Size (nm) | PdI | FALT (nm) |
|---|---|---|---|---|
| Ag NPs | −27.9 ± 0.96 | 32.02 ± 0.69 | 0.6 ± 0.06 | - |
| PCL NPs | −11.5 ± 0.24 | 228.1 ± 0.3 | 0.36 ± 0.03 | - |
| PCL-Ag NPs | −25.4 ± 0.15 | 274.8 ± 0.25 | 0.57 ± 0.01 | 1.82 ± 0.02 |
| HC-NPs | 10.8 ± 0.88 | 286.5 ± 0.56 | 0.62 ± 0.05 | 2.55 ± 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortese, B.; D’Amone, S.; Testini, M.; Ratano, P.; Palamà, I.E. Hybrid Clustered Nanoparticles for Chemo-Antibacterial Combinatorial Cancer Therapy. Cancers 2019, 11, 1338. https://doi.org/10.3390/cancers11091338
Cortese B, D’Amone S, Testini M, Ratano P, Palamà IE. Hybrid Clustered Nanoparticles for Chemo-Antibacterial Combinatorial Cancer Therapy. Cancers. 2019; 11(9):1338. https://doi.org/10.3390/cancers11091338
Chicago/Turabian StyleCortese, Barbara, Stefania D’Amone, Mariangela Testini, Patrizia Ratano, and Ilaria Elena Palamà. 2019. "Hybrid Clustered Nanoparticles for Chemo-Antibacterial Combinatorial Cancer Therapy" Cancers 11, no. 9: 1338. https://doi.org/10.3390/cancers11091338
APA StyleCortese, B., D’Amone, S., Testini, M., Ratano, P., & Palamà, I. E. (2019). Hybrid Clustered Nanoparticles for Chemo-Antibacterial Combinatorial Cancer Therapy. Cancers, 11(9), 1338. https://doi.org/10.3390/cancers11091338