An In Vitro Evaluation of the Molecular Mechanisms of Action of Medical Plants from the Lamiaceae Family as Effective Sources of Active Compounds against Human Cancer Cell Lines
Abstract
:Simple Summary
Abstract
1. Introduction
2. Criteria for Selection of Experimental Papers
3. Cancer
4. Cancer and Plants
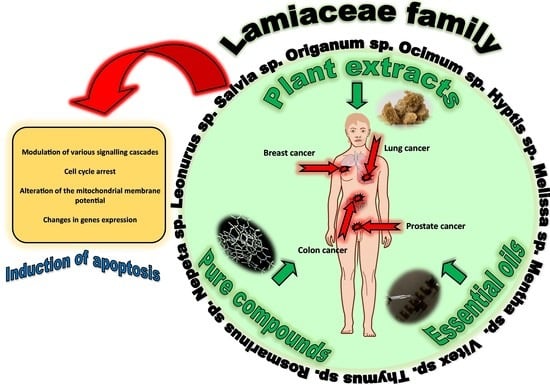
5. The Lamiaceae Family Plants
5.1. The Lamiaceae Family as a Source of Valuable Secondary Metabolites with Anti-Cancer Potential
5.2. The Anti-Cancer Activity of Plant Extracts from the Lamiaceae Family
5.2.1. The Activity of Plant Extracts from the Lamiaceae Family as Modulators of Cell Cycle
5.2.2. The Activity of Plant Extracts from the Lamiaceae Family as Modulators of Apoptosis Signaling
5.2.3. The Activity of Plant Extracts from the Lamiaceae Family as Modulators of p53 Signaling
5.2.4. The Activity of Plant Extracts from the Lamiaceae Family as Modulators of PI3K/AKT Signaling
5.2.5. The Activity of Plant Extracts from the Lamiaceae Family as Modulators of NF-κB Signaling
5.2.6. The Activity of Plant Extracts from the Lamiaceae Family as Modulators of Wnt/β-catenin Signaling
5.2.7. The Activity of Plant Extracts from the Lamiaceae Family as Modulators of Autophagy Signaling
5.2.8. The Activity of Plant Extracts from the Lamiaceae Family as Modulators of Necrosis Signaling
5.2.9. Plant Extracts from the Lamiaceae family and their Impact on Angiogenesis
5.3. The Anti-Cancer Activity of Plant-Derived Compounds from the Lamiaceae Family
5.3.1. The Anticancer Activity of Phenolics Compounds from the Lamiaceae Family
5.3.2. The Anticancer Activity of Terpenoids Compounds from the Lamiaceae Family
5.3.3. The Anticancer Activity of Polysaccharides Compounds from the Lamiaceae Family
5.4. The Anticancer Activity of Essential Oils from the Lamiaceae Family
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Akt | Protein Kinase B |
| ANG | Angiogenin |
| Apaf | Apoptotic Protease Activating Factor |
| Atg | Autophagy-Related Protein |
| Cdc25C | Cell Division Cycle 25C Phosphatase |
| CDKs | Cyclin-Dependent Kinases |
| COX | Cyclooxygenase |
| Deptor | DEP-Domain-Containing mTOR-Interacting Protein |
| DISC | Death-Inducing Signaling Complex |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| ERK | Extracellular Signal-Regulated Kinase |
| FADD | Fas-Associated Death Domain |
| Fz receptor | Frizzled Receptor |
| HIF | Hypoxia-Inducible Factor |
| hTERT | Human Telomerase Reverse Transcriptase |
| IKK | IkB Kinase |
| IL | Interleukin |
| LC3 | Autophagosomal Membrane-Associated Protein Light Chain 3 |
| MAPK | Mitogen-Activated Protein Kinase |
| mLST8 | Mammalian Lethal with Sec13 Protein 8 |
| MMP | Matrix Metallopeptidase |
| mTOR | Mammalian Target of Rapamycin |
| mTORC1 | Mammalian Target of Rapamycin Complex 1 |
| NF-κB | Nuclear Factor Kappa B |
| PARP | Poly(ADP-ribose) Polymerase |
| PI3K | Phosphatidylinositol 3-Kinase |
| PI3KC3 | Class IIIPI3K Complex 1 |
| PI3P | Phospatydyloinositol-3-Posphate |
| PRAS40 | Proline-Rich AKT Substrate 40 kDa |
| PTEN | Phosphatase and Tensin Homolog |
| RANTES | Regulated Upon Activation, Normal T-cell Expressed and Secreted |
| Raptor | Regulatory-Associated Protein of mTOR |
| Rheb | Ras Homolog Enriched in Brain |
| ROS | Reactive Oxygen Species |
| SHP2 | Src Homology Phosphotyrosine Phosphatase 2 |
| SIRT1 | Sirtuin |
| TAK | TGF-β-Activated Kinase |
| TNF | Tumor Necrosis Factor |
| TRAIL | TNF-Related Apoptosis-Inducing Ligand |
| ULK1 | Unc-51-like Kinase 1 Complex |
| VEGF | Vascular Endothelial Growth Factor |
References
- Cooper, G.M. Cancer. In The Cell. A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer development, progression, and therapy: An epigenetic overview. Int. J. Mol. Sci. 2013, 14, 21087–21113. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO—CancerReport—2020—Global Profile. Available online: https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=4-cancer-country-profiles-2020&alias=51561-global-cancer-profile-2020&Itemid=270&lang=fr (accessed on 27 August 2020).
- Voda, A.I.; Bostan, I. Public Health Care Financing and the Costs of Cancer Care: A Cross-National Analysis. Cancers 2018, 10, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.; Fuentes, F.; Lee, J.H.; Tony Kong, A.H. Plants Against Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anticancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Rana, C.S. Plant secondary metabolites: A review. Int. J. Eng. Res. Gen. Sci. 2015, 3, 661–670. [Google Scholar]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Goodman, S.N.; Samet, J. Cause and Cancer Epidemiology. In Cancer Epidemiology and Prevention, 2nd ed.; Schottenfeld, D., Fraumeni, J.F., Eds.; Oxford University Press: New York, NY, USA, 2017. [Google Scholar]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef] [Green Version]
- Binder, M.; Roberts, C.; Spencer, N.; Antoine, D.; Cartwright, C. On the antiquity of cancer: Evidence for metastatic carcinoma in a young man from ancient Nubia (c. 1200 BC). PLoS ONE 2014, 9, e90924. [Google Scholar] [CrossRef] [Green Version]
- The Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 27 August 2020).
- Vineis, P.; Wild, C.P. Global cancer patterns: Causes and prevention. Lancet 2014, 383, 549–557. [Google Scholar] [CrossRef]
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Thun, M.J.; DeLancey, J.O.; Center, M.M.; Jemal, A.; Ward, E.M. The global burden of cancer: Priorities for prevention. Carcinogenesis. 2010, 31, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Møller, B. Predicting the future burden of cancer. Nat. Rev. Cancer. 2006, 6, 63–74. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global epidemiology of lung cancer. Ann. Glob. Health. 2019, 85, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Malvezzi, M.; Bosetti, C.; Rosso, T.; Bertuccio, P.; Chatenoud, L.; Levi, F.; Romano, C.; Negri, E.; la Vecchia, C. Lung cancer mortality in European men: Trends and predictions. Lung Cancer 2013, 80, 138–145. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, Y.C.; Hung, R.J.; Boffetta, P.; Xie, D.; Wampfler, J.A.; Cote, M.L.; Chang, S.C.; Ugolini, D.; Neri, M.; et al. Secondhand Tobacco Smoke Exposure and Lung Adenocarcinoma In Situ/Minimally Invasive Adenocarcinoma (AIS/MIA). Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1902–1906. [Google Scholar] [CrossRef] [Green Version]
- Boffetta, P.; Pershagen, G.; Jöckel, K.H.; Forastiere, F.; Gaborieau, V.; Heinrich, J.; Jahn, I.; Kreuzer, M.; Merletti, F.; Nyberg, F.; et al. Cigar and pipe smoking and lung cancer risk: A multicenter study from Europe. J. Natl. Cancer Inst. 1999, 91, 697–701. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, A.G.; Cote, M.L. Epidemiology of Lung Cancer. Adv. Exp. Med. Biol. 2016, 893, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Carper, M.B.; Claudio, P.P. Clinical potential of gene mutations in lung cancer. Clin. Transl. Med. 2015, 4, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zendehdel, M.; Niakan, B.; Keshtkar, A.; Rafiei, E.; Salamat, F. Subtypes of benign breast disease as a risk factor for breast cancer: A systematic review and meta-analysis protocol. Iran. J. Med. Sci. 2018, 43, 1–8. [Google Scholar] [PubMed]
- George, B.P.; Abrahamse, H. A Review on Novel Breast Cancer Therapies: Photodynamic Therapy and Plant Derived Agent Induced Cell Death Mechanisms. Anticancer Agents Med Chem. 2016, 16, 793–801. [Google Scholar] [CrossRef]
- Malone, K.E.; Daling, J.R.; Thompson, J.D.; O’Brien, C.A.; Francisco, L.V.; Ostrander, E.A. BRCA1 Mutations and Breast Cancer in the General Population. JAMA 1998, 279, 922–929. [Google Scholar] [CrossRef]
- Haber, D. Prophylactic oophorectomy to reduce the risk of ovarian and breast cancer in carriers of BRCA mutations. N. Engl. J. Med. 2002, 346, 1660–1662. [Google Scholar] [CrossRef]
- Armstrong, N.; Ryder, S.; Forbes, C.; Ross, J.; Quek, R.G.W. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin. Epidemiol. 2019, 11, 543–561. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Miki, Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004, 95, 11. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Wen, L.; Wang, Y.; Chen, B.; Xue, Y.; Guo, L.; Liao, N. Impact of TP53 mutations in breast cancer: Clinicopathological features and prognosisImpact of TP53 mutations in breast CA. Thorac. Cancer 2020, 11, 1861–1868. [Google Scholar] [CrossRef]
- Taitt, H.E. Global Trends and Prostate Cancer: A Review of Incidence, Detection, and Mortality as Influenced by Race, Ethnicity, and Geographic Location. Am. J. Mens Health 2018, 12, 1807–1823. [Google Scholar] [CrossRef] [Green Version]
- Rawla, P. Epidemiology of Prostate Cancer. Med. Nucl. 2008, 32, 2–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuzick, J.; Thorat, M.A.; Andriole, G.; Brawley, O.W.; Brown, P.W.; Culig, Z.; Eeles, R.A.; Ford, L.G.; Hamdy, F.C.; Holmberg, L.; et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014, 15, e484–e492. [Google Scholar] [CrossRef] [Green Version]
- Attard, G.; Parker, C.; Eeles, R.A.; Schröder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Bashir, M.N.; Ahmad, M.R.; Malik, A. Risk factors of prostate cancer: A case-control study in Faisalabad, Pakistan. Asian Pac. J. Cancer Prev. 2014, 15, 10237–10240. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Chen, Y.; Nguyen, D.T.; Thompson, Z.J.; Eroshkin, A.M.; Nerlakanti, N.; Patel, A.K.; Agarwal, N.; Teer, J.K.; Dhillon, J.; et al. The Homeobox gene, HOXB13, Regulates a Mitotic Protein-Kinase Interaction Network in Metastatic Prostate Cancers. Sci. Rep. 2019, 9, 9715. [Google Scholar] [CrossRef] [Green Version]
- Wallis, C.J.D.; Nam, R.K. Prostate Cancer Genetics: A Review. EJIFCC 2015, 26, 79–91. [Google Scholar]
- Dong, J.T. Prevalent mutations in prostate cancer. J. Cell. Biochem. 2006, 97, 433–447. [Google Scholar] [CrossRef]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular basis of colorectal cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef] [Green Version]
- Kheirelseid, E.A.H.; Miller, N.; Kerin, M.J. Molecular biology of colorectal cancer: Review of the literature. Am. J. Mol. Biol. 2013, 3, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Benarba, B.; Pandiella, A. Colorectal cancer and medicinal plants: Principle findings from recent studies. Biomed. Pharmacother. 2018, 107, 408–423. [Google Scholar] [CrossRef]
- Watson, A.J.M.; Collins, P.D. Colon cancer: A civilization disorder. Dig. Dis. 2011, 29, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D. Epidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancer. Diabetes Care 2013, 36 (Suppl. 2), S233–S239. [Google Scholar] [CrossRef] [Green Version]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephenson, W. History of Medicine. Br. Med. J. 1957, 2, 413. [Google Scholar] [CrossRef]
- Koul, B. Herbs for Cancer Treatment, 1st ed.; Springer: New York, NY, USA, 2019. [Google Scholar]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016, 30, 1243–1352. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 1, 982–991. [Google Scholar] [CrossRef] [Green Version]
- Sitarek, P.; Merecz-Sadowska, A.; Kowalczyk, T.; Wieczfinska, J.; Zajdel, R.; Śliwiński, T. Potential synergistic action of bioactive compounds from plant extracts against skin infecting microorganisms. Int. J. Mol. Sci. 2020, 21, 5105. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef] [Green Version]
- Zielinska-Blizniewska, H.; Sitarek, P.; Merecz-Sadowska, A.; Malinowska, K.; Zajdel, K.; Jablonska, M.; Sliwinski, T.; Zajdel, R. Plant extracts and reactive oxygen species as two counteracting agents with anti- and pro-obesity properties. Int. J. Mol. Sci. 2019, 20, 4556. [Google Scholar] [CrossRef] [Green Version]
- Seca, A.M.L.; Pinto, D.C.G.A. Biological Potential and Medical Use of Secondary Metabolites. Medicines 2019, 6, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines (Basel) 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Wink, M. Functions and Biotechnology of Plant Secondary Metabolites, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2010. [Google Scholar]
- Rajeswara Rao, B.; Pandu Sastry, K.; Kumar Kothari, S. Cultivation technology for economically important medicinal plants. In Advances in Medicinal Plants, 1st ed.; Reddy, K.J., Bahadur, B., Bhadraiah, B., Rao, M.L.N., Eds.; Universities Press: Hyderabad, India, 2007. [Google Scholar]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwell, M.; Rahman, P.K.S.M. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Synowiec, E.; Kowalczyk, T.; Śliwiński, T.; Skała, E. An In Vitro Estimation of the Cytotoxicity and Genotoxicity of Root Extract from Leonurus sibiricus L. Overexpressing AtPAP1 against Different Cancer Cell Lines. Molecules 2018, 23, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skała, E.; Sitarek, P.; Toma, M.; Szemraj, J.; Radek, M.; Nieborowska-Skorska, M.; Skorski, T.; Wysokińska, H.; Śliwiński, T. Inhibition of human glioma cell proliferation by altered Bax/Bcl-2-p53 expression and apoptosis induction by Rhaponticum carthamoides extracts from transformed and normal roots. J. Pharm. Pharmacol. 2016, 68, 1454–1464. [Google Scholar] [CrossRef]
- Sitarek, P.; Kowalczyk, T.; Santangelo, S.; Białas, A.J.; Toma, M.; Wieczfinska, J.; Śliwiński, T.; Skała, E. The Extract of Leonurus sibiricus Transgenic Roots with AtPAP1 Transcriptional Factor Induces Apoptosis via DNA Damage and Down Regulation of Selected Epigenetic Factors in Human Cancer Cells. Neurochem. Res. 2018, 43, 1363–1370. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, T.; Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Pytel, D.; Wieczfińska, J.; Szemraj, J.; Śliwiński, T. Induction of apoptosis by in vitro and in vivo plant extracts derived from Menyanthes trifoliata L. in human cancer cells. Cytotechnology 2019, 71, 165–180. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, T.; Sitarek, P.; Toma, M.; Picot, L.; Wielanek, M.; Skała, E.; Śliwiński, T. An Extract of Transgenic Senna obtusifolia L. hairy roots with Overexpression of PgSS1 Gene in Combination with Chemotherapeutic Agent Induces Apoptosis in the Leukemia Cell Line. Biomolecules 2020, 10, 510. [Google Scholar] [CrossRef] [Green Version]
- Gali-Muhtasib, H.; Hmadi, R.; Kareh, M.; Tohme, R.; Darwiche, N. Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis 2015, 20, 1531–1562. [Google Scholar] [CrossRef]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.; Chai, H.B.; Kinghorn, A.D. Discovery of new anticancer agents from higher plants. Front. Biosci. (Schol Ed.) 2012, 4, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carović-Stanko, K.; Petek, M.; Grdiša, M.; Pintar, J.; Bedeković, D.; Ćustić, M.H.; Satovic, Z. Medicinal plants of the family Lamiaceae as functional foods-A review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Bekut, M.; Brkić, S.; Kladar, N.; Dragović, G.; Gavarić, N.; Božin, B.B. Potential of selected Lamiaceae plants in anti(retro) viral therapy. Pharmacol. Res. 2018, 133, 301–314. [Google Scholar] [CrossRef]
- Stankovic, M. Lamiaceae Species, 1st ed.; MDPI: Basel, Switzerland, 2020. [Google Scholar]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action. Biomed. Res. Int. 2018, 2018, 4154185. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Wei, Z.; Zhang, S.; Peng, X.; Huang, Y.; Zhang, Y.; Lu, J. Phenolic Fractions from Muscadine Grape “Noble” Pomace can Inhibit Breast Cancer Cell MDA-MB-231 Better than those from European Grape “Cabernet Sauvignon” and Induce S-Phase Arrest and Apoptosis. J. Food Sci. 2017, 82, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Omodanisi, E.I.; Aboua, Y.G.; Oguntibeju, O.O. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of moringa oleifera in diabetes-induced nephrotoxic male wistar rats. Molecules 2017, 22, 439. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; Tomás-Barberán, F.A.; Espín, J.C. Neuroprotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef] [Green Version]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; Gatta, D.M.P.; Patruno, A.; De Lutiis, M.A.; Quiles, J.L.; Grilli, A.; Felaco, M.; Speranza, L. Biological effect of licochalcone C on the regulation of PI3K/Akt/eNOS and NF-κB/iNOS/NO signaling pathways in H9c2 cells in response to LPS stimulation. Int. J. Mol. Sci. 2017, 18, 690. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Parvez, M.K.; Arbab, A.H.; Al-Dosari, M.S. Quantitative analysis of rutin, quercetin, naringenin, and gallic acid by validated RP- and NP-HPTLC methods for quality control of anti-HBV active extract of Guiera senegalensis. Pharm. Biol. 2017, 55, 1317–1323. [Google Scholar] [CrossRef] [Green Version]
- Ayub, M.A.; Hussain, A.I.; Hanif, M.A.; Chatha, S.A.S.; Kamal, G.M.; Shahid, M.; Janneh, O. Variation in Phenolic Profile, β-Carotene and Flavonoid Contents, Biological Activities of Two Tagetes Species from Pakistani Flora. Chem. Biodivers. 2017, 14, e1600463. [Google Scholar] [CrossRef]
- Miyamoto, T.; Zhang, X.; Ueyama, Y.; Apisada, K.; Nakayama, M.; Suzuki, Y.; Ozawa, T.; Mitani, A.; Shigemune, N.; Shimatani, K.; et al. Development of novel monoclonal antibodies directed against catechins for investigation of antibacterial mechanism of catechins. J. Microbiol. Methods 2017, 137, 6–13. [Google Scholar] [CrossRef]
- D’Archivio, M.; Santangelo, C.; Scazzocchio, B.; Varì, R.; Filesi, C.; Masella, R.; Giovannini, C. Modulatory effects of polyphenols on apoptosis induction: Relevance for cancer prevention. Int. J. Mol. Sci. 2008, 9, 213–228. [Google Scholar] [CrossRef]
- Thomas-Charles, C.; Fennell, H. Anti-Prostate Cancer Activity of Plant-Derived Bioactive Compounds: A Review. Curr. Mol. Biol. Rep. 2019, 5, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Perveen, S. Introductory Chapter: Terpenes and Terpenoids. In Terpenes and Terpenoids, 1st ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2016, 33, 1227–1238. [Google Scholar] [CrossRef] [Green Version]
- Hanson, J.R.; Nichols, T.; Mukhrish, Y.; Bagley, M.C. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2019, 36, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Topçu, G.; Yücer, R.; Şenol, H. Bioactive constituents of Anatolian Salvia species. In Salvia Biotechnology, 1st ed.; Georgiev, V., Atanas, P., Eds.; Springer: New York, NY, USA, 2018. [Google Scholar]
- Bisio, A.; Pedrelli, F.; D’Ambola, M.; Labanca, F.; Schito, A.M.; Govaerts, R.; De Tommasi, N.; Milella, L. Quinone diterpenes from Salvia species: Chemistry, botany, and biological activity. Phytochem. Rev. 2019, 18, 665–842. [Google Scholar] [CrossRef]
- Demetzos, C.; Dimas, K.S. Labdane-type diterpenes: Chemistry and biological activity. Stud. Nat. Prod. Chem. 2001, 25, 235–292. [Google Scholar] [CrossRef]
- Banerjee, A.; Laya, M.; Mora, H.; Cabrera, E. The Chemistry of Bioactive Diterpenes. Curr. Org. Chem. 2008, 12, 1050–1070. [Google Scholar] [CrossRef]
- Khattak, S.; Khan, H. Anti-cancer Potential of Phyto-alkaloids: A Prospective Review. Curr. Cancer Ther. Rev. 2016, 12, 66–75. [Google Scholar] [CrossRef]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Aniszewski, T. Alkaloids–Secrets of Life: Alkaloid Chemistry, Biological Significance, Applications and Ecological Role, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Burnet, M.W.; Goldmann, A.; Message, B.; Drong, R.; El Amrani, A.; Loreau, O.; Slightom, J.; Tepfer, D. The stachydrine catabolism region in Sinorhizobium meliloti encodes a multi-enzyme complex similar to the xenobiotic degrading systems in other bacteria. Gene 2000, 244, 151–161. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer activity of essential oils: A review. J. Sci. Food Agric. 2013, 93, 3643–3653. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Oluwaseun Ademiluyi, A.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Sitarek, P.; Rijo, P.; Garcia, C.; Skała, E.; Kalemba, D.; Białas, A.J.; Szemraj, J.; Pytel, D.; Toma, M.; Wysokińska, H.; et al. Antibacterial, Anti-Inflammatory, Antioxidant, and Antiproliferative Properties of Essential Oils from Hairy and Normal Roots of Leonurus sibiricus L. and Their Chemical Composition. Oxid. Med. Cell Longev. 2017, 2017, 7384061. [Google Scholar] [CrossRef] [Green Version]
- Yousef, I.; Oran, S.; Bustanji, Y.; Al Eisawi, D.; Irmaileh, B.A. Cytotoxic Effect of Selected Wild Medicinal Plant Species from Jordan on Two Different Breast Cancer Cell Lines, MCF7 and T47D. Biol. Med. 2018, 10, 4. [Google Scholar] [CrossRef]
- Özdemir, A.; Yildiz, M.; Senol, F.S.; Şimay, Y.D.; Ibişoglu, B.; Gokbulut, A.; Orhan, I.E.; Ark, M. Promising anticancer activity of Cyclotrichium niveum L. extracts through induction of both apoptosis and necrosis. Food Chem. Toxicol. 2017, 109, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, S.; Delazar, A.; Asgharian, P.; Lotfipour, F.; Moghaddam, S.B.; Afshar, F.H. In-vitro bioactivity and phytochemical screening of extracts from rhizomes of eremostachys azerbaijanica rech. F. Growing in Iran. Iran. J. Pharm. Res. 2017, 16, 306–314. [Google Scholar]
- Hoshyar, R.; Mostafavinia, S.E.; Zarban, A.; Hassanpour, M.; Partovfari, M.; Taheri, A.; Pouyan, M. Correlation of Anticancer Effects of 12 Iranian Herbs on Human breast Adenocarcinoma cells with antioxidant Properties. Free Radic. Antioxid. 2015, 5, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Amalina, N.D.; Suzery, M.; Cahyono, B. Cytotoxic Activity of Hyptis Pectinate Extracts on MCF-7 Human Breast Cancer Cells. Indones. J. Cancer Chemoprevent. 2020, 11, 1–6. [Google Scholar] [CrossRef]
- Santana, F.R.; Luna-Dulcey, L.; Antunes, V.U.; Tormena, C.F.; Cominetti, M.R.; Duarte, M.C.; da Silva, J.A. Evaluation of the cytotoxicity on breast cancer cell of extracts and compounds isolated from Hyptis pectinata (L.) poit. Nat. Prod. Res. 2020, 34, 102–109. [Google Scholar] [CrossRef]
- Al-Sheddi, E.S. Cytotoxic potential of Petroleum ether, ethyl acetate, chloroform, and ethanol extracts of Lavandula Coronopifolia against human breast carcinoma cell line (MDA-MB-321). Asian Pac. J. Cancer Prev. 2019, 20, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Yusufoglu, H.; Foudah, A.I.; Alqarni, M.; Alam, A.; Salkini, A.; Ahmed, E.O. Phenolic contents, cytotoxicity and antimicrobial activity of five medicinal plants of the lamiaceae family obtained from Saudi Arabia local markets. Indo Am. J. Pharm. Sci. 2019, 06, 14418–14425. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Modaeinama, S.; Abasi, M.; Abbasi, M.M.; Jahanban-Esfahlan, R. Anti proliferative properties of Melissa officinalis in different human cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 5703–5707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, D.B.; Castro, I.; Lopes-Rodrigues, V.; Pereira, J.M.; Barros, L.; Ferreira, I.C.F.R.; Xavier, C.P.R.; Vasconcelos, M.H. Melissa officinalis L. ethanolic extract inhibits the growth of a lung cancer cell line by interfering with the cell cycle and inducing apoptosis. Food Funct. 2018, 9, 3134–3142. [Google Scholar] [CrossRef] [Green Version]
- Zengin, G.; Ferrante, C.; Gnapi, D.E.; Sinan, K.I.; Orlando, G.; Recinella, L.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Chiavaroli, A.; et al. Comprehensive approaches on the chemical constituents and pharmacological properties of flowers and leaves of American basil (Ocimum americanum L). Food Res. Int. 2019, 125, 108610. [Google Scholar] [CrossRef] [PubMed]
- Abdelhady, M.I.S.; Motaal, A.A. A cytotoxic C-glycosylated derivative of apigenin from the leaves of Ocimum basilicum var. thyrsiflorum. Braz. J. Pharmacogn. 2016, 26, 763–766. [Google Scholar] [CrossRef] [Green Version]
- Makrane, H.; El Messaoudi, M.; Melhaoui, A.; El Mzibri, M.; Benbacer, L.; Aziz, M. Cytotoxicity of the Aqueous Extract and Organic Fractions from Origanum majorana on Human Breast Cell Line MDA-MB-231 and Human Colon Cell Line HT-29. Adv. Pharmacol. Sci. 2018, 2018, 3297193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldisi, S.S.; Jaganjac, M.; Eid, A.H.; Goktepe, I. Evaluation of Apoptotic, Antiproliferative, and Antimigratory Activity of Origanum syriacum against Metastatic Colon Cancer Cells. J. Herbs Spices Med. Plants 2019, 25, 202–217. [Google Scholar] [CrossRef]
- Pobba, R.; Rama Kotaiah, M.; Chandra Sekar, K.B. Evaluation of antioxidant and anticancer activities of Orthosiphon aristatus (Blume). Int. J. Res. Pharm. Sci. 2015, 6, 193–198. [Google Scholar]
- Singh, M.K.; Dhongade, H.; Tripathi, D.K. Orthosiphon pallidus, a potential treatment for patients with breast cancer. J. Pharmacopunct. 2017, 20, 265–273. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Budovsky, A.; Ben-Shabat, S.; Khalfin, B.; Gorelick, J.; Bishitz, Y.; Miloslavski, R.; Yarmolinsky, L. Recent Updates on the Phytochemistry and Pharmacological Properties of Phlomis viscosa Poiret. Rejuvenation Res. 2019, 22, 282–288. [Google Scholar] [CrossRef]
- Yulianto, W.; Andarwulan, N.; Giriwono, P.E.; Pamungkas, J. HPLC-based metabolomics to identify cytotoxic compounds from Plectranthus amboinicus (Lour.) Spreng against human breast cancer MCF-7Cells. J. Chromatogr. 2016, 1039, 28–34. [Google Scholar] [CrossRef]
- Rai, V.; Pai, V.R.; Kedilaya, P. A preliminary evaluation of anticancer and antioxidant potential of two traditional medicinal plants from lamiaceae-pogostemon heyneanus and plectranthus amboinicus. J. Appl. Pharm. Sci. 2016, 6, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Borrás-Linares, I.; Pérez-Sánchez, A.; Lozano-Sánchez, J.; Barrajón-Catalán, E.; Arráez-Román, D.; Cifuentes, A.; Micol, V.; Carretero, A.S. A bioguided identification of the active compounds that contribute to the antiproliferative/cytotoxic effects of rosemary extract on colon cancer cells. Food Chem. Toxicol. 2015, 80, 215–222. [Google Scholar] [CrossRef] [Green Version]
- El-burai, H.R. Cytotoxic and Antiproliferative Effects of Four Natural Plants Extracts on Colon Cancer Caco-2 Cell Line. Master’s Thesis, The Islamic University, Gaza, Palestine, 2017. [Google Scholar]
- Marrelli, M.; Cristaldi, B.; Menichini, F.; Conforti, F. Inhibitory effects of wild dietary plants on lipid peroxidation and on the proliferation of human cancer cells. Food Chem. Toxicol. 2015, 86, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Han, J.; Zheng, X.; Ai, B.; Yang, Y.; Xiao, D.; Zheng, L.; Sheng, Z. Rosemary leaf extract inhibits glycation, breast cancer proliferation, and diabetes risks. Appl. Sci. 2020, 10, 2249. [Google Scholar] [CrossRef] [Green Version]
- Tundis, R.; Iacopetta, D.; Sinicropi, M.S.; Bonesi, M.; Leporini, M.; Passalacqua, N.G.; Ceramella, J.; Menichini, F.; Loizzo, M.R. Assessment of antioxidant, antitumor and pro-apoptotic effects of Salvia fruticosa Mill. subsp. thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi (Lamiaceae). Food Chem. Toxicol. 2017, 106, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Campos-Xolalpa, N.; Alonso-Castro, Á.J.; Sánchez-Mendoza, E.; Zavala-Sánchez, M.Á.; Pérez-Gutiérrez, S. Cytotoxic activity of the chloroform extract and four diterpenes isolated from Salvia ballotiflora. Braz. J. Pharmacogn. 2017, 27, 302–305. [Google Scholar] [CrossRef]
- Eltawaty, S.I.; Yagoub, S.O.; Shouman, S.A.; Ahmed, A.; Omer, F.A. Anticancer Effects of Methanol Extract of Libyan Salvia Fruticosa Mill on Mcf7, T47D and (Mda-Mb-468) Breast Cells Lines. Eur. J. Pharm. Med. Res. 2020, 7, 165–169. [Google Scholar]
- Altay, A.; Kılıc Suloglu, A.; Sagdıcoglu Celep, G.; Selmanoglu, G.; Bozoglu, F. Anatolıan sage Salvıa frutıcosa ınhıbıts cytosolıc glutathıone-s-transferase actıvıty and colon cancer cell prolıferatıon. J. Food Meas. Charact. 2019, 13, 1390–1399. [Google Scholar] [CrossRef]
- Güzel, S.; Ülger, M.; Özay, Y. Antimicrobial and Antiproliferative Activities of Chia (Salvia hispanica L.) Seeds. Int. J. Second. Metab. 2020, 7, 174–180. [Google Scholar] [CrossRef]
- Kumar, D.G.; Perumal, P.C.; Kumar, K.; Muthusami, S.; Gopalakrishnan, V.K. Dietary evaluation, antioxidant and cytotoxic activity of crude extract from chia seeds (Salvia hispanica L.) against human prostate cancer cell line (PC-3). Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1358–1362. [Google Scholar]
- Garcia, C.S.C.; Menti, C.; Lambert, A.P.F.; Barcellos, T.; Moura, S.; Calloni, C.; Branco, C.S.; Salvador, M.; Roesch-Ely, M.; Henriques, J.A.P. Pharmacological perspectives from Brazilian Salvia officinalis (Lamiaceae): Antioxidant, and antitumor in mammalian cells. An. Acad. Bras. Cienc. 2016, 88, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Darwish, A.; Hamad, G.; Sohaimy, S. Nutrients and Constituents Relevant to Antioxidant, Antimicrobial and Anti-Breast Cancer Properties of Salvia officinalis L. Int. J. Biochem. Res. Rev. 2018, 23, 1–13. [Google Scholar] [CrossRef]
- Yumrutaş, Ö.; Pehlivan, M.; Güven, C.; Bozgeyik, İ.; Bozgeyik, E.; Temiz, E.; Yumrutaş, P.; Üçkardeş, F. Investigation of Cytotoxic Effect of Salvia pilifera Extracts and Synthetic Chlorogenic and Caffeic Acids on DU145 Prostate Cancer Cells Line. KSU J. Agric Nat. 2018, 21, 141–147. [Google Scholar] [CrossRef]
- Güzel, S.; Kahraman, A.; Ülger, M.; Özay, Y.; Bozgeyik, İ.; Sarikaya, Ö. Morphology, myxocarpy, mineral content and in vitro antimicrobial and antiproliferative activities of mericarps of the vulnerable Turkish endemic Salvia pilifera. J. Res. Pharm. 2019, 23, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Al-Zereini, W.A. Ononis natrix and Salvia verbenaca: Two Jordanian Medicinal Plants with Cytotoxic and Antibacterial Activities. J. Herbs Spices Med. Plants. 2017, 23, 18–25. [Google Scholar] [CrossRef]
- Yfanti, P.; Batistatou, A.; Manos, G.; Lekka, M.E. The Aromatic Plant Satureja horvatii ssp. macrophylla Induces Apoptosis and Cell Death to the A549 Cancer Cell Line. Am. J. Plant Sci. 2015, 6, 2092–2103. [Google Scholar] [CrossRef] [Green Version]
- Demirelma, H.; Gelinci, E. Determination of the cytotoxic effect on human colon cancer and phe nolic substance cont ent of the endemic species sideritis ozturkii Aytaç & Aksoy. Appl. Ecol. Environ. Res. 2019, 17, 7407–7419. [Google Scholar] [CrossRef]
- Yumrutas, O.; Oztuzcu, S.; Pehlivan, M.; Ozturk, N.; Eroz Poyraz, I.; Iğci, Y.Z.; Cevik, M.O.; Bozgeyik, I.; Aksoy, A.F.; Bagis, H.; et al. Cell viability, anti-proliferation and antioxidant activities of Sideritis syriaca, Tanacetum argenteum sub sp. argenteum and Achillea aleppica subsp. zederbaueri on human breast cancer cell line (MCF-7). J. Appl. Pharm. Sci. 2015, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Montaño, J.M.; Martínez-Sánchez, S.M.; Burgos-Morón, E.; Guillén-Mancina, E.; Jiménez-Alonso, J.J.; García, F.; Aparicio, A.; López-Lázaro, M. Screening for Selective Anticancer Activity of 65 Extracts of Plants Collected in Western Andalusia, Spain. Preprints 2018, 2018060177. [Google Scholar] [CrossRef] [Green Version]
- Sadeghisamani, F.; Sazgar, H.; Ghasemi Pirbalouti, A. Investigation Cytotoxic Effect of Hydroalcholic Extract from Combination of Kelussia odaratissma Mozaff and Thymus daenesis Celak on MCF-7 Cancer Cells Line. J. Jahrom Univ. Med Sci. 2016, 14, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Garbi, M.I.; Osman, E.E.; Kabbashi, A.S.; Saleh, M.S.; Yuosof, Y.S.; Mahmoud, S.A.; Salam, H.A.A. Cytotoxicity of Vitex trifolia leaf extracts on MCF-7 and Vero cell lines. J. Sci. Innov. Res. 2015, 4, 89–93. [Google Scholar]
- Abu-Gharbieh, E.; El-Huneidi, W.; Shehab, N.G.; Bajbouj, K.; Vinod, A.; El-Serafi, A.; Malhab, L.B.; Abdel-Rahman, W.M. Anti-tumor activity of the ethanolic extract of Micromeria fruticosa on human breast and colon cancer cells. FASEB J. 2020, 34. [Google Scholar] [CrossRef]
- El-Huneidi, W.; Shehab, N.G.; Bajbouj, K.; Vinod, A.; El-serafi, A.; Shafarin, J.; Boumalhab, L.J.; Abdel-rahman, W.M.; Abu-gharbieh, E. Micromeriafruticosa induces cell cycle arrest and apoptosis in breast and colorectal cancer cells. Pharmaceuticals 2020, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Weidner, C.; Rousseau, M.; Plauth, A.; Wowro, S.J.; Fischer, C.; Abdel-Aziz, H.; Sauer, S. Melissa officinalis extract induces apoptosis and inhibits proliferation in colon cancer cells through formation of reactive oxygen species. Phytomedicine 2015, 22, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Song, H.M.; Park, G.H.; Park, S.B.; Kim, H.S.; Son, H.J.; Um, Y.; Jeong, J.B. Vitex rotundifolia Fruit Suppresses the Proliferation of Human Colorectal Cancer Cells through Down-regulation of Cyclin D1 and CDK4 via Proteasomal-Dependent Degradation and Transcriptional Inhibition. Am. J. Chin. Med. 2018, 46, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A. In vitro antioxidant and antiproliferative activities of six international basil cultivars. Nat. Prod. Res. 2015, 29, 2149–2154. [Google Scholar] [CrossRef]
- Ye, Y.T.; Zhong, W.; Sun, P.; Wang, D.; Wang, C.; Hu, L.M.; Qian, J.Q. Apoptosis induced by the methanol extract of Salvia miltiorrhiza Bunge in non-small cell lung cancer through PTEN-mediated inhibition of PI3K/Akt pathway. J. Ethnopharmacol. 2017, 200, 107–116. [Google Scholar] [CrossRef]
- Gao, W.; Xu, H.L.Y.L.Y.L.Y. Root extract of Prunella vulgaris inhibits in vitro and in vivo carcinogenesis in MCF-5 human breast carcinoma via suppression of angiogenesis, induction of apoptosis, cell cycle arrest and modulation of PI3K/AKT signalling pathway. J. BUON 2019, 24, 549–554. [Google Scholar]
- Khojasteh, A.; Metón, I.; Camino, S.; Cusido, R.M.; Eibl, R.; Palazon, J. In Vitro Study of the Anticancer Effects of Biotechnological Extracts of the Endangered Plant Species Satureja Khuzistanica. Int. J. Mol. Sci. 2019, 20, 2400. [Google Scholar] [CrossRef] [Green Version]
- Sridevi, M.; Bright, J.; Yamini, K. Anti-cancer effect of ocimum-sanctum ethanolic extract in non-small cell lung carcinoma cell line. Int. J. Pharm. Pharm. Sci. 2016, 8, 8–20. [Google Scholar]
- Chen, C.C.; Kao, C.P.; Chiu, M.M.; Wang, S.H. The anti-cancer effects and mechanisms of Scutellaria barbata D. Don on CL1-5 lung cancer cells. Oncotarget 2017, 8, 109340–109357. [Google Scholar] [CrossRef] [PubMed]
- Kokhdan, E.P.; Sadeghi, H.; Ghafoori, H.; Sadeghi, H.; Danaei, N.; Javadian, H.; Aghamaali, M.R. Cytotoxic effect of methanolic extract, alkaloid and terpenoid fractions of Stachys pilifera against HT-29 cell line. Res. Pharm. Sci. 2018, 13, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Sicora, O.; Naghi, M.A.; Soos, I.; Sicora, C. The ethanolic stem extract of Caryopteris x Clandonensis posseses antiproliferative potential by blocking breast cancer cells in mitosis. Farmacia 2019, 67, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Panicker, N.G.; Balhamar, S.O.M.S.; Akhlaq, S.; Qureshi, M.M.; Rizvi, T.S.; AlHarrasi, A.; Hussain, J.; Mustafa, F. Identification and Characterization of the Caspase-Mediated Apoptotic Activity of Teucrium mascatense and an Isolated Compound in Human Cancer Cells. Molecules 2019, 24, 977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadok, V.A.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grujičić, D.; Marković, A.; Vukajlović, J.T.; Stanković, M.; Jakovljević, M.R.; Ćirić, A.; Djordjević, K.; Planojević, N.; Milutinović, M.; Milošević-Djordjević, O. Genotoxic and cytotoxic properties of two medical plants (Teucrium arduini L.and Teucrium flavum L.) in relation to their polyphenolic contents. Mutat. Res. 2020, 852, 503168. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, G.E.S.; Jan, R.; Mohamad, H.; Tengku Muhammad, T. Vitex rotundifolia fractions induce apoptosis in human breast cancer cell line, MCF-7, via extrinsic and intrinsic pathways. Res. Pharm. Sci. 2019, 14, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, S.; Azad, H.; Rathinavelu, A. Apoptosis Induction by Ocimum sanctum Extract in LNCaP Prostate Cancer Cells. J. Med. Food. 2015, 18, 776–785. [Google Scholar] [CrossRef]
- Geryani, M.A.; Mahdian, D.; Mousavi, S.H.; Hosseini, A. Ctotoxic and apoptogenic effects of Perovskia abrotanoides flower extract on MCF-7 and HeLa cell lines. Avicenna J. Phytomed. 2016, 6, 410–417. [Google Scholar]
- Golshan, A.; Amini, E.; Emami, S.A.; Asili, J.; Jalali, Z.; Sabouri-Rad, S.; Sanjar-Mousavi, N.; Tayarani-Najaran, Z. Cytotoxic evaluation of different fractions of Salvia chorassanica Bunge on MCF-7 and du 145 cell lines. Res. Pharm. Sci. 2016, 11, 73–80. [Google Scholar]
- Tarhan, L.; Nakipoğlu, M.; Kavakcıoğlu, B.; Tongul, B.; Nalbantsoy, A. The Induction of Growth Inhibition and Apoptosis in HeLa and MCF-7 Cells by Teucrium sandrasicum, Having Effective Antioxidant Properties. Appl. Biochem. Biotechnol. 2016, 178, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Arab, F. Antiproliferative activity of Origanum compactum extract on lung cancer and hepatoma cells. Arab. J. Med. Aromat. Plants. 2015, 1, 44–56. [Google Scholar] [CrossRef]
- Kim, H.I.; Hong, S.H.; Ku, J.M.; Lim, Y.S.; Lee, S.J.; Song, J.; Kim, T.Y.; Cheon, C.; Ko, S.G. Scutellaria radix promotes apoptosis in non-small cell lung cancer cells via induction of AMPK-dependent autophagy. Am. J. Chin. Med. 2019, 47, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, R.; Seidi, K.; Monfaredan, A.; Shafie-Irannejad, V.; Abbasi, M.M.; Karimian, A.; Yousefi, B. The herbal medicine Melissa officinalis extract effects on gene expression of p53, Bcl-2, Her2, VEGF-A and hTERT in human lung, breast and prostate cancer cell lines. Gene 2017, 613, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Laila, F.; Fardiaz, D.; Yuliana, N.D.; Damanik, M.R.M.; Nur Annisa Dewi, F. Methanol Extract of Coleus amboinicus (Lour) Exhibited Antiproliferative Activity and Induced Programmed Cell Death in Colon Cancer Cell WiDr. Int. J. Food Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, R.; Pemaiah, B.; Sridharan, S.; Narayanan, M.; Ramalingam, S. Enhanced cytotoxic potential of Orthosiphon stamineus extract in MCF-7 cells through suppression of nucleolin and BCL2. Bangladesh J. Pharmacol. 2017, 12, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J.; Lee, S.; Choi, N.R.; Jo, S.H.; Cho, S. Dracocephalum palmatum Stephan on human-derived prostate cancer cell death. Kor. J. Herbology. 2018, 33, 69–76. [Google Scholar] [CrossRef]
- Milutinović, M.G.; Maksimović, V.M.; Cvetković, D.M.; Nikodijević, D.D.; Stanković, M.S.; Pešić, M.; Marković, S.D. Potential of Teucrium chamaedrys L. to modulate apoptosis and biotransformation in colorectal carcinoma cells. J. Ethnopharmacol. 2019, 240, 111951. [Google Scholar] [CrossRef]
- Benhalilou, N.; Alsamri, H.; Alneyadi, A.; Athamneh, K.; Alrashedi, A.; Altamimi, N.; Dhaheri, Y.A.; Eid, A.H.; Iratni, R. Origanum majorana ethanolic extract promotes colorectal cancer cell death by triggering abortive autophagy and activation of the extrinsic apoptotic pathway. Front. Oncol. 2019, 9, 795. [Google Scholar] [CrossRef]
- Emami, S.A.; Asili, J.; HosseinNia, S.; Yazdian-Robati, R.; Sahranavard, M.; Tayarani-Najaran, Z. Growth inhibition and apoptosis induction of essential oils and extracts of Nepeta cataria L. on human prostatic and breast cancer cell lines. Asian Pac. J. Cancer Prev. 2016, 17, 125–130. [Google Scholar] [CrossRef]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of Poly(ADP-ribose) Polymerase (PARP) Cleavage in Apoptosis. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.; Megaly, M.; MacNeil, A.J.; Klentrou, P.; Tsiani, E. Rosemary extract reduces Akt/mTOR/p70S6K activation and inhibits proliferation and survival of A549 human lung cancer cells. Biomed. Pharmacother. 2016, 83, 725–732. [Google Scholar] [CrossRef]
- Wise, J.F.; Berkova, Z.; Mathur, R.; Zhu, H.; Braun, F.K.; Tao, R.H.; Sabichi, A.L.; Ao, X.; Maeng, H.; Samaniego, F. Nucleolin inhibits Fas ligand binding and suppresses Fas-mediated apoptosis in vivo via a surface nucleolin-Fas complex. Blood 2013, 121, 4729–4739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wattanathamsan, O.; Hayakawa, Y.; Pongrakhananon, V. Molecular mechanisms of natural compounds in cell death induction and sensitization to chemotherapeutic drugs in lung cancer. Phyther. Res. 2019, 33, 2531–2547. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.e.S. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, P.K.; Goel, A.; Mittal, R.D. Survivin: A molecular biomarker in cancer. Indian J. Med. Res. 2015, 141, 389–397. [Google Scholar] [CrossRef]
- Wei, L.H.; Lin, J.M.; Chu, J.F.; Chen, H.W.; Li, Q.Y.; Peng, J. Scutellaria barbata D. Don inhibits colorectal cancer growth via suppression of Wnt/β-catenin signaling pathway. Chin. J. Integr. Med. 2017, 23, 858–863. [Google Scholar] [CrossRef]
- Castedo, M.; Ferri, K.F.; Kroemer, G. Mammalian Target of Rapamycin (mTOR): Pro- and Anti-Apoptotic. Cell Death Differ. 2002, 9, 99–100. [Google Scholar] [CrossRef]
- Torres, R.G.; Casanova, L.; Carvalho, J.; Marcondes, M.C.; Costa, S.S.; Sola-Penna, M.; Zancan, P. Ocimum basilicum but not Ocimum gratissimum present cytotoxic effects on human breast cancer cell line MCF-7, inducing apoptosis and triggering mTOR/Akt/p70S6K pathway. J. Bioenerg. Biomembr. 2018, 50, 93–105. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Chang, F.; Lee, J.T.; Navolanic, P.M.; Steelman, L.S.; Shelton, J.G.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia 2003, 17, 590–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Bao, Y.; Meng, X.; Wang, S.; Li, T.; Chang, X.; Yang, G.; Bo, T. Mechanism of modulation through PI3K-AKT pathway about Nepeta cataria L.’s extract in non-small cell lung cancer. Oncotarget 2017, 8, 31395–31405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Bai, L.; Chen, W.; Xu, S. The NF-κB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin. Ther. Targets. 2010, 14, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliviero, M.; Romilde, I.; Beatrice, M.M.; Matteo, V.; Giovanna, N.; Consuelo, A.; Claudio, C.; Giorgio, S.; Maggi, F.; Massimo, N. Evaluations of thyme extract effects in human normal bronchial and tracheal epithelial cell lines and in human lung cancer cell line. Chem. Biol. Interact. 2016, 256, 125–133. [Google Scholar] [CrossRef]
- Bakhle, Y.S.; Botting, R.M. Cyclooxygenase-2 and its regulation in inflammation. Mediators Inflamm. 1996, 5, 305–323. [Google Scholar] [CrossRef]
- Uzunhisarcikli, E.; Gürbüz, P.; Yerer, M.B. Investigation of Antiinflamatory Effects of Origanum majorana L. Extract in LPS-Induced Beas-2b and A549 Cells. Proceedings 2019, 40, 8. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell. 2009, 17, 9–26. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Reguart, N.; You, L.; Mazieres, J.; Xu, Z.; Lee, A.Y.; Mikami, I.; McCormick, F.; Jablons, D.M. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005, 24, 3054–3058. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and cancer metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef] [Green Version]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [Green Version]
- Morales, J.C.; Li, L.; Fattah, F.J.; Dong, Y.; Bey, E.A.; Patel, M.; Gao, J.; Boothman, D.A. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit. Rev. Eukaryot. Gene Expr. 2014, 21, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Zong, W.X.; Thompson, C.B. Necrotic death as a cell fate. Genes Dev. 2006, 20, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Henriquez, M.; Armisen, R.; Stutzin, A.; Quest, A.F.G. Cell Death by Necrosis, a Regulated Way to go. Curr. Mol. Med. 2008, 8, 187–206. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Agulló-Chazarra, L.; Herranz-López, M.; Valdés, A.; Cifuentes, A.; Micol, V. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci. Rep. 2019, 9, 808. [Google Scholar] [CrossRef]
- Ghiulai, R.; Avram, S.; Stoian, D.; Pavel, I.Z.; Coricovac, D.; Oprean, C.; Vlase, L.; Farcas, C.; Mioc, M.; Minda, D.; et al. Lemon Balm Extracts Prevent Breast Cancer Progression in Vitro and in Ovo on Chorioallantoic Membrane Assay. Evid. Based Complement. Altern. Med. 2020, 2020, 6489159. [Google Scholar] [CrossRef] [Green Version]
- Van Cruijsen, H.; Giaccone, G.; Hoekman, K. Epidermal growth factor receptor and angiogenesis: Opportunities for combined anticancer strategies. Int. J. Cancer. 2005, 117, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor Angiogenesis: From Bench to Bedside. In Tumor Angiogenesis: Basic Mechanisms and Cancer Therapy, 1st ed.; Marmé, D., Fusenig, N., Eds.; Springer: New York, NY, USA, 2008; pp. 3–28. [Google Scholar]
- Atmaca, H.; Bozkurt, E. Apoptotic and anti-angiogenic effects of Salvia triloba extract in prostate cancer cell lines. Tumor Biol. 2016, 37, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi, Z.; Khazaei, M. Leptin and its cardiovascular effects: Focus on angiogenesis. Adv. Biomed. Res. 2015, 4, 79. [Google Scholar] [CrossRef]
- Suffee, N.; Richard, B.; Hlawaty, H.; Oudar, O.; Charnaux, N.; Sutton, A. Angiogenic properties of the chemokine RANTES/CCL5. Biochem. Soc. Trans. 2011, 39, 1649–1653. [Google Scholar] [CrossRef] [Green Version]
- Shestenko, O.P.; Nikonov, S.D.; Mertvetsov, N.P. Angiogenin and its functions in angiogenesis. Mol. Biol. 2001, 35, 294–314. [Google Scholar] [CrossRef]
- Begley, L.A.; Kasina, S.; Mehra, R.; Adsule, S.; Admon, A.J.; Lonigro, R.J.; Chinnaiyan, A.M.; Macoska, J.A. CXCL5 promotes prostate cancer progression. Neoplasia 2008, 10, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.M.; Chou, G.X.; Yang, Q.S.; Wang, W.; Zhou, J.L. Abietane diterpenoids from Caryopteris incana (Thunb.) Miq. Org. Biomol. Chem. 2016, 14, 3510–3520. [Google Scholar] [CrossRef]
- Luo, Y.; Cheng, L.Z.; Luo, Q.; Yan, Y.M.; Wang, S.M.; Sun, Q.; Cheng, Y.X. New ursane-type triterpenoids from Clerodendranthus spicatus. Fitoterapia 2017, 119, 69–74. [Google Scholar] [CrossRef]
- Somwong, P.; Suttisri, R. Cytotoxic activity of the chemical constituents of Clerodendrum indicum and Clerodendrum villosum roots. J. Integr. Med. 2018, 16, 57–61. [Google Scholar] [CrossRef]
- Ba Vinh, L.; Thi Minh Nguyet, N.; Young Yang, S.; Hoon Kim, J.; Thi Vien, L.; Thi Thanh Huong, P.; Van Thanh, N.; Xuan Cuong, N.; Hoai Nam, N.; Van Minh, C.; et al. A new rearranged abietane diterpene from Clerodendrum inerme with antioxidant and cytotoxic activities. Nat. Prod. Res. 2018, 32, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, S.; Jia, O.; Zhu, Q.; Shi, L. Bioactive diterpenoids from clerodendrum kiangsiense. Molecules 2016, 21, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awouafack, M.D.; Aimaiti, S.; Tane, P.; Morita, H. Clerodendrumol, a new triterpenoid from Clerodendrum yaundense Gürke (Lamiaceae). Helv. Chim. Acta 2016, 99, 161–164. [Google Scholar] [CrossRef]
- Dai, L.P.; Li, C.; Yang, H.Z.; Lu, Y.Q.; Yu, H.Y.; Gao, H.M.; Wang, Z.M. Three new cytotoxic ent-kaurane diterpenes from isodon excisoides. Molecules 2015, 20, 17544–17556. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Zhang, L.X.; Wu, H.; Chen, S.Q.; Li, J.; Dai, L.P.; Wang, Z.M. Four New ent-Kaurane Diterpene Glycosides from Isodon henryi. Molecules 2019, 24, 2736. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, W.G.; Wu, H.Y.; Du, X.; Li, X.N.; Li, Y.; Pu, J.X.; Sun, H.D. Bioactive Enmein-Type ent-Kaurane Diterpenoids from Isodon phyllostachys. J. Nat. Prod. 2016, 79, 132–140. [Google Scholar] [CrossRef]
- Luo, G.Y.; Deng, R.; Zhang, J.J.; Ye, J.H.; Pan, L.T. Two cytotoxic 6,7-seco-spiro-lacton-ent-kauranoids from Isodon rubescens. J. Asian Nat. Prod. Res. 2018, 20, 227–233. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wang, W.G.; Du, X.; Yang, J.; Pu, J.X.; Sun, H.D. Six new cytotoxic and anti-inflammatory 11, 20-epoxy-ent-kaurane diterpenoids from Iso Isodon wikstroemioides. Chin. J. Nat. Med. 2015, 13, 383–389. [Google Scholar] [CrossRef]
- Peng, W.; Huo, G.; Zheng, L.; Xiong, Z.; Shi, X.; Peng, D. Two new oleanane derivatives from the fruits of Leonurus japonicus and their cytotoxic activities. J. Nat. Med. 2019, 73, 252–256. [Google Scholar] [CrossRef]
- To, D.C.; Hoang, D.T.; Tran, M.H.; Pham, M.Q.; Huynh, N.T.; Nguyen, P.H. PTP1B Inhibitory Flavonoids From Orthosiphon stamineus Benth. and Their Growth Inhibition on Human Breast Cancer Cells. Nat. Prod. Commun. 2020, 15, 1934578X19899517. [Google Scholar] [CrossRef]
- Sajjadi, S.; Delazari, Z.; Aghaei, M.; Ghannadian, M. Flavone constituents of Phlomis bruguieri Desf. with cytotoxic activity against MCF-7 breast cancer cells. Res. Pharm. Sci. 2018, 13, 422–429. [Google Scholar] [PubMed]
- Le, D.D.; Nguyen, D.H.; Zhao, B.T.; Kim, J.A.; Kim, S.K.; Min, B.S.; Choi, J.S.; Woo, M.H. 28-Noroleanane-derived spirocyclic triterpenoids and iridoid glucosides from the roots of Phlomoides umbrosa (Turcz.) Kamelin & Makhm with their cytotoxic effects. Phytochemistry 2018, 153, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Amina, M.; Alam, P.; Parvez, M.K.; Al-Musayeib, N.M.; Al-Hwaity, S.A.; Al-Rashidi, N.S.; Al-Dosari, M.S. Isolation and validated HPTLC analysis of four cytotoxic compounds, including a new sesquiterpene from aerial parts of Plectranthus cylindraceus. Nat. Prod. Res. 2018, 32, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, N.; Costa, J.G.; Reis, C.; Almeida, N.; Rijo, P.; Fernandes, A.S. Anti-migratory and pro-apoptotic properties of parvifloron D on triple-negative breast cancer cells. Biomolecules 2020, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Matias, D.; Nicolai, M.; Saraiva, L.; Pinheiro, R.; Faustino, C.; Diaz Lanza, A.; Pinto Reis, C.; Stankovic, T.; Dinic, J.; Pesic, M.; et al. Cytotoxic Activity of Royleanone Diterpenes from Plectranthus madagascariensis Benth. ACS Omega 2019, 4, 8094–8103. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Rakainsa, S.K.; Nisa, K.; Morita, H. Three new abietane-type diterpenoids from the leaves of Indonesian Plectranthus scutellarioides. Fitoterapia 2018, 127, 146–150. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Tran, L.T.T.; Ho, D.V.; Le, D.V.; Raal, A.; Morita, H. Pogostemins A-C, three new cytotoxic meroterpenoids from Pogostemon auricularius. Fitoterapia 2018, 130, 100–104. [Google Scholar] [CrossRef]
- Kim, K.H.; Beemelmanns, C.; Clardy, J.; Cao, S. A new antibacterial octaketide and cytotoxic phenylethanoid glycosides from Pogostemon cablin (Blanco) Benth. Bioorg. Med. Chem. Lett. 2015, 25, 2834–2836. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Mohammed, R.; Hassan, H.M.; Owis, A.I.; Rateb, M.E.; Khanfar, M.A.; Krischke, M.; Mueller, M.J.; Abdelmohsen, U.R. Metabolomic profiling and cytotoxic tetrahydrofurofuran lignans investigations from Premna odorata Blanco. Metabolites 2019, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.Q.; Xuan, L.J. Ent-6,7-Secokaurane diterpenoids from Rabdosia serra and their cytotoxic activities. Phytochemistry 2016, 122, 119–125. [Google Scholar] [CrossRef]
- Esquivel, B.; Bustos-Brito, C.; Sánchez-Castellanos, M.; Nieto-Camacho, A.; Ramírez-Apan, T.; Joseph-Nathan, P.; Quijano, L. Structure, absolute configuration, & antiproliferative activity of abietane & icetexane diterpenoids from salvia ballotiflora. Molecules 2017, 22, 1690. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, H.H.; Firuzi, O.; Baldwin, I.T.; Jassbi, A.R. Cytotoxic activities of different iranian solanaceae and lamiaceae plants and bioassay-guided study of an active extract from salvia lachnocalyx. Nat. Prod. Commun. 2017, 12, 1563–1566. [Google Scholar] [CrossRef] [Green Version]
- Farimani, M.M.; Taleghani, A.; Aliabadi, A.; Aliahmadi, A.; Esmaeili, M.A.; Sarvestani, N.N.; Khavasi, H.R.; Smieško, M.; Hamburger, M.; Ebrahimi, S.N. Labdane diterpenoids from Salvia leriifolia: Absolute configuration, antimicrobial and cytotoxic activities. Planta Med. 2016, 82, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Li, L.W.; Qi, Y.Y.; Liu, S.X.; Wu, X.D.; Zhao, Q.S. Neo-clerodane and abietane diterpenoids with neurotrophic activities from the aerial parts of Salvia leucantha Cav. Fitoterapia 2018, 127, 367–374. [Google Scholar] [CrossRef]
- de Oliveira, P.F.; Munari, C.C.; Nicolella, H.D.; Veneziani, R.C.S.; Tavares, D.C. Manool, a Salvia officinalis diterpene, induces selective cytotoxicity in cancer cells. Cytotechnology 2016, 68, 2139–2143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mofidi Tabatabaei, S.; Salehi, P.; Moridi Farimani, M.; Neuburger, M.; De Mieri, M.; Hamburger, M.; Nejad-Ebrahimi, S. A nor-diterpene from Salvia sahendica leaves. Nat. Prod. Res. 2017, 31, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Jan, T.; Qadri, R.; Naqvi, B.; Adhikari, A.; Nadeem, S.; Muhammad, A. A novel Salvialactomine from the callus culture of Salvia santolinifolia Boiss. Nat. Prod. Res. 2018, 32, 749–754. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Emami, S.A.; Tayarani-Najaran, Z.; Iranshahi, M.; Shakeri, A.; Hohmann, J.; Asili, J. Cytotoxic diterpene quinones from Salvia tebesana Bunge. Fitoterapia 2018, 128, 97–101. [Google Scholar] [CrossRef]
- Fan, M.; Bao, Y.; Zhang, Z.J.; Zhang, H.B.; Zhao, Q.S. New neo-clerodane diterpenoids with neurotrophic activity from the aerial parts of Salvia tiliifolia. Fitoterapia 2017, 123, 44–50. [Google Scholar] [CrossRef]
- Farimani, M.M.; Bahadori, M.B.; Koulaei, S.A.; Salehi, P.; Ebrahimi, S.N.; Khavasi, H.R.; Hamburger, M. New ursane triterpenoids from Salvia urmiensis Bunge: Absolute configuration and anti-proliferative activity. Fitoterapia 2015, 106, 1–6. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Hu, P.; Ji, J.; Li, X.; Chen, J. Neoclerodane diterpenoids from Scutellaria barbata with cytotoxic activities. Nat. Prod. Res. 2020, 34, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.C.; Hu, J.H.; Li, B.L.; Liu, H.; Wang, J.Y.; Sun, L.X. Six New neo -Clerodane Diterpenoids from Aerial Parts of Scutellaria barbata and Their Cytotoxic Activities. Planta Med. 2018, 84, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, C.; Chen, Y.; Li, X.; Chen, J. Cytotoxic Neo-Clerodane Diterpenoids from Scutellaria barbata D.Don. Chem. Biodivers. 2019, 16, e1800499. [Google Scholar] [CrossRef]
- Hanh, T.T.H.; Anh, D.H.; Quang, T.H.; Trung, N.Q.; Thao, D.T.; Cuong, N.T.; An, N.T.; Cuong, N.X.; Nam, N.H.; Van Kiem, P.; et al. Scutebarbatolides A-C, new neo-clerodane diterpenoids from Scutellaria barbata D. Don with cytotoxic activity. Phytochem. Lett. 2019, 29, 65–69. [Google Scholar] [CrossRef]
- Kurimoto, S.I.; Pu, J.X.; Sun, H.D.; Shibata, H.; Takaishi, Y.; Kashiwada, Y. Acylated neo-clerodane type diterpenoids from the aerial parts of Scutellaria coleifolia Levl. (Lamiaceae). J. Nat. Med. 2016, 70, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.J.; Zhang, L.; Xiao, K.; Han, Q.T. New cytotoxic neo-clerodane diterpenoids from Scutellaria strigillosa. Bioorg. Med. Chem. Lett. 2016, 26, 1750–1753. [Google Scholar] [CrossRef] [PubMed]
- Elmasri, W.A.; Hegazy, M.E.F.; Mechref, Y.; Paré, P.W. Cytotoxic saponin poliusaposide from Teucrium polium. RSC Adv. 2015, 5, 27126–27133. [Google Scholar] [CrossRef]
- Ben Sghaier, M.; Mousslim, M.; Pagano, A.; Ammari, Y.; Luis, J.; Kovacic, H. β-eudesmol, a sesquiterpene from Teucrium ramosissimum, inhibits superoxide production, proliferation, adhesion and migration of human tumor cell. Environ. Toxicol. Pharmacol. 2016, 46, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Han, L.; Zheng, D.; Jin, H.; Gai, C.; Wang, J.; Zhang, H.; Zhang, L.; Fu, H. Dimeric abietane diterpenoids and sesquiterpenoid lactones from Teucrium viscidum. J. Nat. Prod. 2015, 78, 630–638. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Peron, G.; Ferrari, S.; Gandin, V.; Bramucci, M.; Quassinti, L.; Mártonfi, P.; Maggi, F. Phytochemical investigations and antiproliferative secondary metabolites from Thymus alternans growing in Slovakia. Pharm. Biol. 2017, 55, 1162–1170. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.D.; Park, Y.S.; Jin, Y.H.; Park, C.S. Production and applications of rosmarinic acid and structurally related compounds. Appl. Microbiol. Biotechnol. 2015, 99, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Chen, H.-H.; Huang, E.; Zhuang, H.; Li, D.; Ni, F. Rosmarinic acid inhibits stem-like breast cancer through hedgehog and Bcl-2/Bax signaling pathways. Pharmacogn. Mag. 2019, 15, 600–606. [Google Scholar] [CrossRef]
- Tai, M.C.; Tsang, S.Y.; Chang, L.Y.F.; Xue, H. Therapeutic potential of wogonin: A naturally occurring flavonoid. CNS Drug Rev. 2005, 11, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Li, X.; Yang, W.H.; Yong, K. A flavone, Wogonin from Scutellaria baicalensis inhibits the proliferation of human colorectal cancer cells by inducing of autophagy, apoptosis and G2/M cell cycle arrest via modulating the PI3K/AKT and STAT3 signalling pathways. J. BUON 2019, 24, 1143–1149. [Google Scholar] [PubMed]
- Ji-Hyun, G.O.; Wei, J.D.; Park, J.I.; Ahn, K.S.; Kim, J.H. Wogonin suppresses the LPS-enhanced invasiveness of MDA-MB-231 breast cancer cells by inhibiting the 5-LO/BLT2 cascade. Int. J. Mol. Med. 2018, 42, 1899–1908. [Google Scholar] [CrossRef] [Green Version]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef]
- Chledzik, S.; Strawa, J.; Matuszek, K.; Nazaruk, J. Pharmacological Effects of Scutellarin, An Active Component of Genus Scutellaria and Erigeron: A Systematic Review. Am. J. Chin. Med. 2018, 46, 319–337. [Google Scholar] [CrossRef]
- Guo, F.; Yang, F.; Zhu, Y.H. Scutellarein from Scutellaria barbata induces apoptosis of human colon cancer HCT116 cells through the ROS-mediated mitochondria-dependent pathway. Nat. Prod. Res. 2019, 33, 2372–2375. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.M.; Cao, Z.X.; Yan, H.L.; Li, W.; Yang, F.; Zhao, W.J.; Diao, Q.C.; Tan, Y. zhu A new abietane diterpenoid from Ajuga ovalifolia var. calantha induces human lung epithelial A549 cell apoptosis by inhibiting SHP2. Fitoterapia 2020, 141, 104484. [Google Scholar] [CrossRef]
- Shakeri, A.; Delavari, S.; Ebrahimi, S.N.; Asili, J.; Emami, S.A.; Tayarani-Najaran, Z. A new tricyclic abietane diterpenoid from Salvia chloroleuca and evaluation of cytotoxic and apoptotic activities. Braz. J. Pharmacogn. 2019, 29, 30–35. [Google Scholar] [CrossRef]
- Wang, J.N.; Zhang, Z.R.; Che, Y.; Yuan, Z.Y.; Lu, Z.L.; Li, Y.; Li, N.; Wan, J.; Sun, H.D.; Sun, N.; et al. Acetyl-macrocalin B, an ent-kaurane diterpenoid, initiates apoptosis through the ROS-p38-caspase 9-dependent pathway and induces G2/M phase arrest via the Chk1/2-Cdc25C-Cdc2/cyclin B axis in non-small cell lung cancer. Cancer Biol. Ther. 2018, 19, 609–621. [Google Scholar] [CrossRef] [Green Version]
- Cortese, K.; Marconi, S.; D’Alesio, C.; Calzia, D.; Panfoli, I.; Tavella, S.; Aiello, C.; Pedrelli, F.; Bisio, A.; Castagnola, P. The novel diterpene 7β-acetoxy-20-hydroxy-19,20-epoxyroyleanone from Salvia corrugata shows complex cytotoxic activities against human breast epithelial cells. Life Sci. 2019, 232, 116610. [Google Scholar] [CrossRef] [PubMed]
- Zito, C.I.; Kontaridis, M.I.; Fornaro, M.; Feng, G.S.; Bennett, A.M. SHP-2 Regulates the Phosphatidylinositide 3′-Kinase/Akt Pathway and Suppresses Caspase 3-Mediated Apoptosis. J. Cell. Physiol. 2004, 199, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death—Apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]
- Bartek, J.; Lukas, J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003, 3, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.C.; Park, J.E.; Park, B.C.; Kim, J.H.; Jeong, D.G.; Park, S.G.; Cho, S. Cell cycle-dependent Cdc25C phosphatase determines cell survival by regulating apoptosis signal-regulating kinase 1. Cell Death Differ. 2015, 22, 1605–1617. [Google Scholar] [CrossRef] [Green Version]
- Uto, T.; Tung, N.H.; Ohta, T.; Juengsanguanpornsuk, W.; Hung, L.Q.; Hai, N.T.; Long, D.D.; Thuong, P.T.; Okubo, S.; Hirata, S.; et al. Antiproliferative activity and apoptosis induction by trijuganone C isolated from the root of Salvia miltiorrhiza Bunge (Danshen). Phyther. Res. 2018, 32, 657–666. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, X.; Li, S.; Sun, Z.; Yu, J.; Chen, C.; Shen, X.; Pan, W.; Luo, H. A novel tanshinone analog exerts anti-cancer effects in prostate cancer by inducing cell apoptosis, arresting cell cycle at G2 phase and blocking metastatic ability. Int. J. Mol. Sci. 2019, 20, 4459. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Lou, Z.; Zhang, G.; Xu, G.; Zhang, G. Diterpenoid Tanshinones, the extract from Danshen (Radix Salviae Miltiorrhizae) induced apoptosis in nine human cancer cell lines. J. Tradit. Chin. Med. 2016, 36, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Zaker, A.; Asili, J.; Abrishamchi, P.; Tayarani-Najaran, Z.; Mousavi, S.H. Cytotoxic and apoptotic effects of root extract and tanshinones isolated from Perovskia abrotanoides kar. Iran. J. Basic Med. Sci. 2017, 20, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Yao, G.; Zhang, M.; Guo, G.; Hu, Y.; Jiang, J.; Cheng, L.; Shi, J.; Li, H.; Zhang, Y.; et al. A novel ent-kaurane diterpenoid executes antitumor function in colorectal cancer cells by inhibiting Wnt/β-catenin signaling. Carcinogenesis 2014, 36, 318–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahadori, M.B.; Eskandani, M.; De Mieri, M.; Hamburger, M.; Nazemiyeh, H. Anti-proliferative activity-guided isolation of clerodermic acid from Salvia nemorosa L.: Geno/cytotoxicity and hypoxia-mediated mechanism of action. Food Chem. Toxicol. 2018, 120, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B. 2015, 5, 378–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.X.; Ji, X.X.; Bao, J.; Li, Q.Q.; Ji, D.D.; Luo, L. Inhibition of proliferation, migration and invasion of human non-small cell lung cancer cell line a549 by phlomisoside f from phlomis younghusbandii mukerjee. Trop. J. Pharm. Res. 2016, 15, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.L.; Qin, F.; Huang, R.Z.; Liang, D.; Wang, C.G.; Wang, H.S.; Liao, Z.X. NF-κB inhibitory and cytotoxic activities of hexacyclic triterpene acid constituents from Glechoma longituba. Phytomedicine 2019, 63, 153037. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.T.T.; Ho, D.V.; Le, D.V.; Van Phan, K.; Nguyen, H.T.; Raal, A. Apoptosis-Inducing Effect of Pogostemin A Isolated from the Aerial Parts of Pogostemon auricularius Against the Human Lung Cancer Cells. J. Biol. Act. Prod. from Nat. 2019, 9, 320–327. [Google Scholar] [CrossRef]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, extraction and biomedical properties of polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Sun, D.; Wang, X. Effects of Scutellaria barbata polysaccharide on the proliferation, apoptosis and EMT of human colon cancer HT29 Cells. Carbohydr. Polym. 2017, 167, 90–96. [Google Scholar] [CrossRef]
- Li, H.; Su, J.; Jiang, J.; Li, Y.; Gan, Z.; Ding, Y.; Li, Y.; Liu, J.; Wang, S.; Ke, Y. Characterization of polysaccharide from Scutellaria barbata and its antagonistic effect on the migration and invasion of HT-29 colorectal cancer cells induced by TGF-β1. Int. J. Biol. Macromol. 2019, 131, 886–895. [Google Scholar] [CrossRef]
- Basappa, G.; Kumar, V.; Sarojini, B.K.; Poornima, D.V.; Gajula, H.; Sannabommaji, T.K.; Rajashekar, J. Chemical composition, biological properties of Anisomeles indica Kuntze essential oil. Ind. Crops Prod. 2015, 77, 89–96. [Google Scholar] [CrossRef]
- Rigano, D.; Marrelli, M.; Formisano, C.; Menichini, F.; Senatore, F.; Bruno, M.; Conforti, F. Phytochemical profile of three Ballota species essential oils and evaluation of the effects on human cancer cells. Nat. Prod. Res. 2017, 31, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Scharf, D.R.; Simionatto, E.L.; Carvalho, J.E.; Salvador, M.J.; Santos, É.P.; Stefanello, M.É.A. Chemical composition and cytotoxic activity of the essential oils of Cantinoa stricta (Benth.) Harley & J.F.B. Pastore (Lamiaceae). Rec. Nat. Prod. 2015, 10, 257–261. [Google Scholar]
- Zorzetto, C.; Sánchez-Mateo, C.C.; Rabanal, R.M.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Iannarelli, R.; Papa, F.; Maggi, F. Antioxidant activity and cytotoxicity on tumour cells of the essential oil from Cedronella canariensis var. canariensis (L.) Webb & Berthel. (Lamiaceae). Nat. Prod. Res. 2015, 29, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, M.H.O.; Morgan, J.M.S.; Cesca, K.; Flach, A.; de Moura, N.F. Cytotoxic Activity of Cunila angustifolia Essential Oil. Chem. Biodivers. 2020, 17, e1900656. [Google Scholar] [CrossRef] [PubMed]
- Pudziuvelyte, L.; Stankevicius, M.; Maruska, A.; Petrikaite, V.; Ragazinskiene, O.; Draksiene, G.; Bernatoniene, J. Chemical composition and anticancer activity of Elsholtzia ciliata essential oils and extracts prepared by different methods. Ind. Crops Prod. 2017, 107, 90–96. [Google Scholar] [CrossRef]
- Donadu, M.G.; Usai, D.; Mazzarello, V.; Molicotti, P.; Cannas, S.; Bellardi, M.G.; Zanetti, S. Change in Caco-2 cells following treatment with various lavender essential oils. Nat. Prod. Res. 2017, 31, 2203–2206. [Google Scholar] [CrossRef]
- Damasceno, L.M.O.; Silva, A.L.N.; dos Santos, R.F.; Feitosa, T.A.; Viana, L.G.F.; de Oliveira, R.G.; e Silva, M.G.; Rolim, L.A.; Araújo, C.S.; Araújo, E.C.C.; et al. Cytotoxic activity of chemical constituents and essential oil from the leaves of leonotis nepetifolia (Lamiaceae). Rev. Virtual Quim. 2019, 11, 517–528. [Google Scholar] [CrossRef]
- Nikšić, H.; Durić, K.; Sijamić, I.; Korić, E.; Kusturica, J.; Omeragić, E.; Muratovic, S. In vitro antiproliferative activity of Melissa officinalis L. (Lamiaceae) leaves essential oil. Bol. Latinoam. Caribe Plantas Med. Aromat. 2019, 18, 480–491. [Google Scholar] [CrossRef]
- Ouakouak, H.; Benchikha, N.; Hassani, A.; Ashour, M.L. Chemical composition and biological activity of Mentha citrata Ehrh., essential oils growing in southern Algeria. J. Food Sci. Technol. 2019, 56, 5346–5353. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Bakchiche, B.; ALSalamat, H.A.; Rezzoug, M.; Gherib, A.; Flamini, G. Chemical composition, antioxidant, antimicrobial and Antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan atlas. BMC Complement. Altern. Med. 2018, 18, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mothana, R.A.; Nasr, F.A.; Khaled, J.M.; AL-Zharani, M.; Noman, O.M.; Abutaha, N.; Al-Rehaily, A.J.; Almarfadi, O.M.; Kumar, A.; Kurkcuoglu, M. Analysis of chemical composition and assessment of cytotoxic, antimicrobial, and antioxidant activities of the essential oil of meriandra dianthera growing in Saudi Arabia. Molecules 2019, 24, 2647. [Google Scholar] [CrossRef] [Green Version]
- Kahkeshani, N.; Hadjiakhoondi, A.; Navidpour, L.; Akbarzadeh, T.; Safavi, M.; Karimpour-Razkenari, E.; Khanavi, M. Chemodiversity of Nepeta menthoides Boiss. & Bohse. essential oil from Iran and antimicrobial, acetylcholinesterase inhibitory and cytotoxic properties of 1,8-cineole chemotype. Nat. Prod. Res. 2018, 32, 2745–2748. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Ayatollahi, S.A.; Varoni, E.M.; Salehi, B.; Kobarfard, F.; Sharifi-Rad, M.; Iriti, M.; Sharifi-Rad, M. Chemical composition and functional properties of essential oils from Nepeta schiraziana Boiss. Farmacia 2017, 65, 802–812. [Google Scholar]
- Shakeri, A.; Khakdan, F.; Soheili, V.; Sahebkar, A.; Shaddel, R.; Asili, J. Volatile composition, antimicrobial, cytotoxic and antioxidant evaluation of the essential oil from Nepeta sintenisii Bornm. Ind. Crops Prod. 2016, 84, 224–229. [Google Scholar] [CrossRef]
- Fitsiou, E.; Mitropoulou, G.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Bardouki, H.; Panayiotidis, M.I.; Galanis, A.; Kourkoutas, Y.; Chlichlia, K.; et al. Phytochemical profile and evaluation of the biological activities of essential oils derived from the greek aromatic plant species Ocimum basilicum, Mentha spicata, Pimpinella anisum and Fortunella margarita. Molecules 2016, 21, 1069. [Google Scholar] [CrossRef] [Green Version]
- Hajlaoui, H.; Mighri, H.; Aouni, M.; Gharsallah, N.; Kadri, A. Chemical composition and in vitro evaluation of antioxidant, antimicrobial, cytotoxicity and anti-acetylcholinesterase properties of Tunisian Origanum majorana L. essential oil. Microb. Pathog. 2016, 95, 86–94. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Arnold, N.A.; Menichini, F.; Senatore, F. Composition, antibacterial, antioxidant and antiproliferative activities of essential oils from three Origanum species growing wild in Lebanon and Greece. Nat. Prod. Res. 2016, 30, 735–739. [Google Scholar] [CrossRef]
- Elansary, H.O.; Abdelgaleil, S.A.M.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective antioxidant, antimicrobial and anticancer activities of essential oils of horticultural aromatic crops in northern Egypt. BMC Complement. Altern. Med. 2018, 18, 214. [Google Scholar] [CrossRef]
- Mothana, R.A.; Khaled, J.M.; Noman, O.M.; Kumar, A.; Alajmi, M.F.; Al-Rehaily, A.J.; Kurkcuoglu, M. Phytochemical analysis and evaluation of the cytotoxic, antimicrobial and antioxidant activities of essential oils from three Plectranthus species grown in Saudi Arabia. BMC Complement. Altern. Med. 2018, 18, 237. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Gao, Y.; Lai, P.X. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of essential oil from premna microphylla turczaninow. Molecules 2017, 22, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jardak, M.; Elloumi-Mseddi, J.; Aifa, S.; Mnif, S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017, 16, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eftekhari, M.; Ardekani, M.R.S.; Amini, M.; Akbarzadeh, T.; Safavi, M.; Razkenari, E.K.; Khanavi, M. Biological activities of the essential oil and total extract of Salvia macrosiphon Boiss. J. Basic Clin. Pharm. 2017, 8, 82–86. [Google Scholar]
- Privitera, G.; Luca, T.; Castorina, S.; Passanisi, R.; Ruberto, G.; Napoli, E. Anticancer activity of Salvia officinalis essential oil and its principal constituents against hormone-dependent tumour cells. Asian Pac. J. Trop. Biomed. 2019, 9, 24–28. [Google Scholar] [CrossRef]
- Luca, T.; Napoli, E.; Privitera, G.; Musso, N.; Ruberto, G.; Castorina, S. Antiproliferative Effect and Cell Cycle Alterations Induced by Salvia officinalis Essential Oil and Its Three Main Components in Human Colon Cancer Cell Lines. Chem. Biodivers. 2020, 17, e2000309. [Google Scholar] [CrossRef] [PubMed]
- Alimpić, A.; Pljevljakušić, D.; Šavikin, K.; Knežević, A.; Ćurčić, M.; Veličković, D.; Stević, T.; Petrović, G.; Matevski, V.; Vukojević, J.; et al. Composition and biological effects of Salvia ringens (Lamiaceae) essential oil and extracts. Ind. Crops Prod. 2015, 76, 702–709. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sharifi-Rad, M.; Hoseini-Alfatemi, S.M.; Iriti, M.; Sharifi-Rad, M.; Sharifi-Rad, M. Composition, cytotoxic and antimicrobial activities of Satureja intermedia CA Mey essential oil. Int. J. Mol. Sci. 2015, 16, 17812–17825. [Google Scholar] [CrossRef] [Green Version]
- Fitsiou, E.; Anestopoulos, I.; Chlichlia, K.; Galanis, A.; Kourkoutas, I.; Panayiotidis, M.I.; Pappa, A. Antioxidant and antiproliferative properties of the essential oils of Satureja thymbra and Satureja parnassica and their major constituents. Anticancer Res. 2016, 36, 5757–5763. [Google Scholar] [CrossRef] [Green Version]
- Venditti, A.; Bianco, A.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Damiano, S.; Papa, F.; Vittori, S.; Maleci Bini, L.; Giuliani, C.; et al. Phytochemical analysis, biological activity, and secretory structures of Stachys annua (L.) L. subsp. annua (Lamiaceae) from central Italy. Chem. Biodivers. 2015, 12, 1172–1183. [Google Scholar] [CrossRef]
- Shakeri, A.; D’Urso, G.; Taghizadeh, S.F.; Piacente, S.; Norouzi, S.; Soheili, V.; Asili, J.; Salarbashi, D. LC-ESI/LTQOrbitrap/MS/MS and GC–MS profiling of Stachys parviflora L. and evaluation of its biological activities. J. Pharm. Biomed. Anal. 2019, 168, 209–216. [Google Scholar] [CrossRef]
- de Oliveira, P.F.; Alves, J.M.; Damasceno, J.L.; Oliveira, R.A.M.; Júnior Dias, H.; Crotti, A.E.M.; Tavares, D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015, 25, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Bendif, H.; Boudjeniba, M.; Miara, M.D.; Biqiku, L.; Bramucci, M.; Lupidi, G.; Quassinti, L.; Vitali, L.A.; Maggi, F. Essential Oil of Thymus munbyanus subsp. coloratus from Algeria: Chemotypification and in vitro Biological Activities. Chem. Biodivers. 2017, 14, e1600299. [Google Scholar] [CrossRef] [PubMed]
- Janitermi, M.; Nemati, F. Cytotoxic effect of Zataria multiflora on breast cancer cell line (MCF-7) and normal fibroblast cell. Sci. J. 2015, 36, 1895–1904. [Google Scholar]
- Mohammadpour, G.; Tahmasbpour, R.; Noureini, S.K.; Tahmasbpour, E.; Bagherpour, G. In vitro Antimicrobial and Cytotoxicity Assays of Satureja bakhtiarica and Zataria multiflora Essential Oils. Am. J. Phytomed. Clin. Ther. 2015, 3, 502–511. [Google Scholar]
- Saeidi, M.; Asili, J.; Emami, S.A.; Moshtaghi, N.; Malekzadeh-Shafaroudi, S. Comparative volatile composition, antioxidant and cytotoxic evaluation of the essential oil of Zhumeria majdae from south of Iran. Iran. J. Basic Med. Sci. 2019, 22, 80–85. [Google Scholar] [CrossRef]
- Skorić, M.; Gligorijević, N.; Čavić, M.; Todorović, S.; Janković, R.; Ristić, M.; Mišić, D.; Radulović, S. Cytotoxic activity of Nepeta rtanjensis Diklić & Milojević essential oil and its mode of action. Ind. Crops Prod. 2017, 100, 163–170. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Fitsiou, E.; Bouloukosta, E.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Oreopoulou, A.; Papavassilopoulou, E.; Pappa, A.; Chlichlia, K. Extraction, Chemical Composition, and Anticancer Potential of Origanum onites L. Essential Oil. Molecules 2019, 24, 2612. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, M.; Sangral, M.; Arya, K.; Rather, R. Chemical characterization, biological assessment and molecular docking studies of essential oil of Ocimum viride for potential antimicrobial and anticancer activities. bioRxiv. 2018, 390906. [Google Scholar] [CrossRef]
- Özkan, A.; Erdoğan, A. Evaluation of cytotoxic, membrane, and DNA damaging effects of Thymus revolutus célak essential oil on different cancer cells. Turk. J. Med. Sci. 2017, 47, 702–714. [Google Scholar] [CrossRef]
- Russo, A.; Cardile, V.; Graziano, A.C.E.; Avola, R.; Bruno, M.; Rigano, D. Involvement of Bax and Bcl-2 in induction of apoptosis by essential oils of three Lebanese Salvia species in human prostate cancer cells. Int. J. Mol. Sci. 2018, 19, 292. [Google Scholar] [CrossRef] [Green Version]
- Ahani, N.; Sangtarash, M.H.; Alipour Eskanda-Ni, M.; Houshmand, M. Zataria multiflora boiss. Essential oil induce apoptosis in two human colon cancer cell lines (HCT116 & SW48). Iran. J. Public Health 2020, 49, 753–762. [Google Scholar] [PubMed]
- Zhao, Y.; Chen, R.; Wang, Y.; Qing, C.; Wang, W.; Yang, Y. In Vitro and in Vivo Efficacy Studies of Lavender angustifolia Essential Oil and Its Active Constituents on the Proliferation of Human Prostate Cancer. Integr. Cancer Ther. 2017, 16, 215–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipin Dev, M.S.; Menon, D.B. Essential Oil Extracted from Plectranthus amboinicus Induces Apoptosis in the Lung Cancer Cells via Mitochondrial Pathway. Int. J. Pharm. Sci. Drug Res. 2017, 9, 83–89. [Google Scholar] [CrossRef]
- Lu, X.; Yang, L.; Lu, C.; Xu, Z.; Qiu, H.; Wu, J.; Wang, J.; Tong, J.; Zhu, Y.; Shen, J. Molecular Role of EGFR-MAPK Pathway in Patchouli Alcohol-Induced Apoptosis and Cell Cycle Arrest on A549 Cells in Vitro and in Vivo. BioMed Res. Int. 2016, 2016, 4567580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaradat, N.; Al-Maharik, N. Fingerprinting, antimicrobial, antioxidant, anticancer, cyclooxygenase and metabolic enzymes inhibitory characteristic evaluations of Stachys viticina Boiss. essential oil. Molecules 2019, 24, 3880. [Google Scholar] [CrossRef] [Green Version]
- Athamneh, K.; Alneyadi, A.; Alsamri, H.; Alrashedi, A.; Palakott, A.; El-Tarabily, K.A.; Eid, A.H.; Dhaheri, Y.A.; Iratni, R. Origanum majorana essential oil triggers p38 mapk-mediated protective autophagy, apoptosis, and caspase-dependent cleavage of P70S6K in colorectal cancer cells. Biomolecules 2020, 10, 412. [Google Scholar] [CrossRef] [Green Version]
- Justus, B.; Kanunfre, C.C.; Budel, J.M.; de Faria, M.F.; Raman, V.; de Paula, J.P.; Farago, P.V. New insights into the mechanisms of French lavender essential oil on non-small-cell lung cancer cell growth. Ind. Crops Prod. 2019, 136, 28–36. [Google Scholar] [CrossRef]

 ; Inhibition—
; Inhibition—  .
.
 ; Inhibition—
; Inhibition—  .
.
| Name of The Species | Part of the Plant | Type of Extract | Class of Compounds/Compounds Identified in Extract | Cancer Cell Lines | Ref. |
|---|---|---|---|---|---|
| Ajuga chamaepitys subsp. chia (Schreb.) = synonym of Ajuga chia (Schreb) | aerial parts | ethanolic | - | T-47D | [103] |
| Cyclotrichium niveum (Boiss.) Manden. and Scheng | aerial parts | ethyl acetate | gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, gentisic acid, p-coumaric acid, ferulic acid, rutin, luteolin-7 glucoside, quercetin, luteolin, apigenin | MCF-7 | [104] |
| Eremostachys azerbaijanica Rech.f. | rhizomes | methanolic, n-hexane, dichloromethane | fatty acid derivatives and steroids steroids and derivatives, heterocyclic hydrocarbons, sesquiterpenes, and linear alkanes | A549 | [105] |
| Eremostachys azerbaijanica Rech.f. | rhizomes | dichloromethane | steroids and derivatives, heterocyclic hydrocarbons, sesquiterpenes, and linear alkanes | HT-29 | [105] |
| Hymenocrater platystegius Rech.f. | leaves and flowers | aqueous | phenolics | MCF-7 | [106] |
| Hyptis pectinata L. | leaves | ethanolic | - | MCF-7 | [107] |
| Hyptis pectinate L. | leaves, branches, root | ethanolic | pectinolide J, hyptolide, and pectinolide E | MDA-MB-231 | [108] |
| Lavandula coronopifolia Poir. | aerial parts | petroleum ether, ethyl acetate, chloroform, and ethanol | - | MDA-MB-231 | [109] |
| Lavender angustifolia Mill | leaves | methanolic | phenolics, flavonoids, glycosides | MCF-7 | [110] |
| Melissa officinalis L. | leaves | ethanolic | phenolics and flavonoids | MCF-7, PC-3 and A549 | [111] |
| Melissa officinalis L. | leaves | ethanolic | phenolics: 3-(3,4-dihydroxyphenyl)-lactic acid, caftaric acid, caffeic acid hexoside, fertaric acid, caffeic acid, sulphated rosmarinic acid, yunnaneic acid E, lithospermic acid A isomer, chicoric acid, yunnaneic acid F, salvianolic acid C derivative I, salvianolic acid C derivative II, rosmarinic acid hexoside, sagerinic acid, rosmarinic acid, salvianolic acid A, salvianolic acid C derivative III, lithospermic acid A, salvianolic acid A isomer, salvianolic acid C derivative IV | NCI-H460 | [112] |
| Nepeta bracteata Benth. | flowering shoot | aqueous | phenolics | MCF-7 | [106] |
| Ocimum americanum L. | leaves | ethyl acetate | phenolics, flavonoids, flavanols, tannins, saponins | HCT-116 | [113] |
| Ocimum basilicum var. thyrsiflorum (L.) Benth. | leaves | ethanolic | phenolics and flavonoids: cinnamic acid, gallic acid, methylgallate, ellagic acid, methyl ellagic acid, apigenin, luteolin, vitexin, isovitexin | HCT-116 | [114] |
| Origanum dayi Post. | aerial parts | ethanolic | - | MCF-7 and T47D | [103] |
| Origanum majorana L. | aerial parts | ethyl acetate | 2-(4-hydroxy phenyl) ethanol, vanillic acid, 4-hydroxybenzoic acid, syringic acid, caffeic acid, vanillin, trans-ferulic acid, luteolin, cinnamic acid | MDA-MB-231 and HT-29 | [115] |
| Origanum syriacum L. | leaves | ethanolic | - | LoVo and SW620 | [116] |
| Orthosiphon aristatus (Blume) Miq. | leaves | methanolic | phenolics | MCF-7 | [117] |
| Orthosiphon pallidus Royle ex Benth. | whole plants | aqueous | - | MCF-7 and MDA-MB-231 | [118] |
| Phlomis viscosa Poiret. | leaves, flowers and stems | ethanolic | diosmin, isovaleraldehyde, 2,4-hexadienal, 2-hexenal, alpha terpinene, 1-octen-3-ol, himachala-2,4-diene, n-octanal, buorbonene, 1-propanal, 2-methyl, cubebene | MCF-7 | [119] |
| Plectranthus amboinicus (Lour.) Spreng | leaves | chloroform | diterpene/7-acetoxy-6-hydroxyroyleanone | MCF-7 | [120] |
| Plectranthus amboinicus (Lour.) Spreng. | leaves | ethanolic | - | MCF-7 | [121] |
| Pogostemon heyneanus Benth. | leaves | chloroform | - | MCF-7 and MDA-MB-231 | [121] |
| Rosmarinus officinalis L. | leaves | ethanolic | flavonoids, diterpenes, triterpenes: apigenin, hispidulin, cirsiliol, diosmetin, cirsimaritin, rosmanol, epiisorosmanol, epirosmanol, genkwanin, miltipolone, carnosol, rosmadial, anemosapogenin, rosmaridiphenol, augustic acid, benthamic acid, carnosic acid, 12-methoxycarnosic acid, shogaol, micromeric acid, hinokione, betulinic acid, ursolic acid | HT-29 and SW480 | [122] |
| Rosmarinus officinalis L. | leaves and flowers | aqueous | phenolics | MCF-7 | [106] |
| Rosmarinus officinalis L. | aerial parts | aqueous | - | Caco-2 | [123] |
| Rosmarinus officinalis L. | leaves | methanolic | terpenes | LoVo | [124] |
| Rosmarinus officinalis L. | leaves | methanolic | phenolics and flavonoids, alkaloids, tannins, glycosides | MCF-7 | [110] |
| Rosmarinus officinalis L. | leaves | ethanolic | phenolics: protocatechuic acid, caffeic acid, ellagic acid, ferulic acid, rosemarinic acid, carnosol, carnosic acid | MCF-7 | [125] |
| Salvia fruticosa Mill subsp. thomasii | aerial parts | methanolic | luteolin, luteolin 7-O-glucoside, rutin, salvigenin | MCF-7, MDA-MB-231, RKO and Caco-2 | [126] |
| Salvia ballotiflora Benth. | aerial parts | chloroform | - | A549 | [127] |
| Salvia fruticosa Mill | bark | methanolic | - | MCF-7, T-47D and MDA-468 | [128] |
| Salvia fruticosa Mill. (SF.) | aerial parts | aqueous | phenolics and flavonoids: rosmarinic and caffeic acid | Caco-2 and HT-29 | [129] |
| Salvia hispanica L. | seeds | ethanolic | - | A549 | [130] |
| Salvia hispanica L. | seeds | ethanolic | tannins, saponins, flavonoids, alkaloids, proteins, phenols | PC-3 | [131] |
| Salvia officinalis L. | leaves | ethanolic | eucalyptol (1,8-cineole), α-thujone, β-thujone, camphor, β-caryophyllene, α-caryophyllene (α-humulene), viridiflorol, manool | A-549, HT-29 | [132] |
| Salvia officinalis L. | leaves | aqueous | phenolics: caffeic acid, syringic acid, rutin, coumaric acid, vanillin, quercetin, cinnamic acid | MDA-MB-231 | [133] |
| Salvia pilifera Montbret and Aucher ex Benth. | whole plant | methanolic | fumaric acid, gallic acid, gallocatechin, catechin, oleorufein, 4-hydroxybenzoic acid, caffeic acid, syringic acid, ellagic acid, 3-hydroxy cinnamic acid and protocatechuic acid | DU-145 | [134] |
| Salvia pilifera Montbret et Aucher ex Benth. | mericarps | ethanolic | - | A549 | [135] |
| Salvia verbenaca L. | leaves | ethyl acetate | flavonoids and terpenes | MDA MB-231 | [136] |
| Satureja horvatii subsp. macrophylla (Halácsy) Baden. | aerial parts | methanolic | monoterpene hydrocarbons, oxygenated monoterpens, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, monoterpenes, sesquiterpenes: α-thujene, α-pinene, camphene, 1-octen-3-ol, α-myrcene, 3-octanol, α-phellandrene + δ 3-carene, α-terpinene, p-cymene, limonene, 1,8-cineole, cis-ocimene, γ-terpinene, trans sabinene hydrate, terpinolene, linalool, trans-pinocarveol, cis-verbenol, camphor, borneol, terpinene-4-ol, p-cymen-8-ol, α-terpineol, dihydrocarvone, thymol methyl ether, thymoquinone, thymol, carvacrol, caryophyllene, aromadendrene, α-humulene, alloaromadendrene, γ-muurolene, viridiflorene, γ-elemene, b-bisabolene, γ-cadinene, δ-cadinene, (-)-spathulenol, caryophyllene oxide, viridiflorol | A549 | [137] |
| Sideritis ozturkii Aytaç and Aksoy | flower and leaf | methanolic | gallic acid, protocatechuic acid, catechin, 4-hydroxybenzoic acid, caffeic acid, syringic acid, rutin trihydrate, trans-p-coumaric acid, trans-ferulic acid, myricetin, trans-resveratrol, quercetin, trans-cinnamic acid, naringenin, kaempferol | DLD-11 | [138] |
| Sideritis syriaca L. | leaves | methanolic | phenolic acids: gallic acid, p-hydroxybenzoic acid, cafeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, o-coumaric acid, rosmarinic acid and trans- cinnamic acid | MCF-7 | [139] |
| Teucrium fruticans L. | leaves | ethanol/ethyl acetate/water | - | A549 | [140] |
| Thyme vulgaris L. | leaves | methanolic | phenolics and flavonoids, tannins | MCF-7 | [110] |
| Thymus daenensis Celak. | leaves and stems | ethanolic | - | MCF-7 | [141] |
| Thymus mastichina L. | whole plant | ethanol/ethyl acetate/water | - | A549 | [140] |
| Vitex trifolia L. | leaves | methanol | - | MCF-7 | [142] |
| Name of The Species | Part of the Plant | Active Compounds/ Class of Compounds | Cancer Cell Lines | Ref. |
|---|---|---|---|---|
| Caryopteris incana (Thunb.) Miq., | whole plant | abietane diterpenes: caryopincaolide A, C, D | A549, Calu-1 | [211] |
| Clerodendranthus spicatus (Thunb.) C. Y. Wu | aerial parts | ursane-type triterpenoids: spicatusoids A–E and three known ones and a known oleanane-type triterpenoid | A-549, MCF-7, SW480 | [212] |
| Clerodendrum indicum (L.) Kuntze and Clerodendrum villosum Blume | roots | 3b-hydroxy-D:B-friedo-olean-5-ene, oleanolic acid 3-acetate, taraxerol, lupeol, betulinic acid, (22E)-stigmasta-4,22,25-trien-3-one, stigmasta-4,25-dien-3-one, stigmasta-4,22-dien-3-one, 22-dehydroclerosterol, clerosterol, stigmasterol, b-sitosterol, pectolinarigenin, hispidulin | SW620, ChaGo-K-1, BT474 | [213] |
| Clerodendrum inerme (L.) Gaertn | leaves | harwickiic acid, Crolerodendrum B-abietane diterpenes, uncinatone, acacetin, kaempferol 3,7,4′-trimethyl ether, 14,15-dihydro-15β-methoxy-3-epi-caryoptin, 5α,8α-epidioxyergosta-6,22-diene-3β-ol | HCT-116 | [214] |
| Clerodendrum kiangsiense Merrill ex H. L. Li | herb | abeo-abietane diterpenoid: 12-methoxy-6,11,14,16-tetrahydroxy-17(15Ñ16)-abeo5,8,11,13-abietatetraen-3,7-dione, cryptojaponol, fortunin E | A549, MCF-7 | [215] |
| Clerodendrum yaundense Gurke | twigs | lupane-type triterpene: (16a)-lupa-12,20(29)-dien-16-ol (clerodendrumol) and, O-Acetylclerodendrumol (16a)-Lupa-12,20(29)-dien-16-yl Ace-tate | MDA-MB-231 | [216] |
| Isodon excisoides (Sun ex C. H. Hu) H. Hara, J. Jap | aerial parts | 1α,7α,14β-trihydroxy-20-acetoxy-ent-kaur-15-one, henryin, reniformin C; kamebacetal A | HCT-116, NCI-H1650 | [217] |
| Isodon rubescens (Hemsl.) H. Hara | aerial parts | ent-7,20-epoxy-kaur-16-en-1α,6β,7β,15β-tetrahydroxyl-11-O-β-d-glucopyranoside, ent-7,20-epoxy-kaur-16-en-6β,7β,14β,15β-tetrahydroxyl-1-O-β-d-glucopyranoside, ent-7,20-epoxy-kaur-16-en-6β,7β,15β-trihydroxyl-1-O-β-d-glucopyranoside, andent-7,20-epoxy-kaur-16-en-7β,11β,14α,15β-tetrahydr-oxyl-6-O-β-d-glucopyranoside, sodonterpene II, enmenol-1-β-glucoside, andenmenol | HCT-116 | [218] |
| Isodon phyllostachys (Diels) Kudo | aerial parts | enmein-type diterpenoids enmein-type diterpenoids: 20-episerrin C, serrin C, isodocarpin, serrin B | A549, MCF-7, SW480 | [219] |
| Isodon rubescens (Hemsl.) H.Hara | leaves | diterpenoids: 6-epi-11-O-acetylangustifolin and 11-O-acetylangustifolin | A549 | [220] |
| Isodon wikstroemioides (Hand.–Mazz.) H. Hara | aerial parts | diterpenoids: 11, 20-epoxy-ent- kauranoids, isowikstroemins H–M, along with two known analogues, macrocalyxin B | A549, MCF-7 | [221] |
| Leonurus japonicus Houtt. | fruits | leonuronins A and B | A549 | [222] |
| Ocimum basilicum var.thyrsiflorum (L.) Benth | leaves | C-glycosylated derivative of apigenin: 3′′-O-acetylvitexin | HCT-116 | [114] |
| Ocimum sanctum L. | aerial parts | lignans: (-)-rabdosiin | MCF-7, SKBR3, HCT-116 | [201] |
| Orthosiphon stamineus Benth. | aerial parts | 7,4′-dimethylkaempferol, 5,7,4′-trimethylkaempferol, 7,3′,4′-trimethylquercetin, 5,7,3′,4′-tetramethylquercetin, 5,7,4′-trimethylquercetin, 3,5,3′,4′-tetramethylquercetin, 5,7,3′,6′-tetramethoxyflavone, 5,7,3′,4′-tetramethoxyflavone, 2S-5,6,7,3′,4′-pentamethoxyflavanone, 2S-5′-hydroxy-5,7,3′,4′-tetramethoxyflavanone | MCF-7, MDA-MB-231 | [223] |
| Phlomis bruguieri Desf | aerial parts | 4’-methoxy-luteolin-7-phosphate | MCF-7 | [224] |
| Phlomoides umbrosa (Turcz.) Kamelin & Makhm | roots | 28-noroleanane-derived spirocyclic triterpenoids: phlomisu D, phlomisu E, (2α,3α,17R,18β)-19(18→17)-abeo-28-norolean-12-ene-2,3,18,23,24-pentol, phlomispentaol | MCF-7 | [225] |
| Plectranthus cylindraceus Hoechst. Ex. Benth | aerial parts | sesquiterpene: plectranol A, maaliol, penduletin and chrysosplenol D | MBD-MB-321 | [226] |
| Plectranthus ecklonii Benth. | whole plant | abietane diterpenoid: parvifloron D | MDA-MB-231 | [227] |
| Plectranthus madagascariensis Benth | whole plant | royleanone diterpenes: 7α-formyloxy-6β-hydroxyroyleanon, 7α,6β-dihydroxyroyleanon, 7α-acetoxy-6β-hydroxyroy-leanone | MDA-MB-231, MCF-7, HCT-116, NCI-H460 | [228] |
| Plectranthus scutellarioides (L.) R. Br. | leaves | diterpenoids: spiroscutelones A–C | MCF-7 | [229] |
| Pogostemon auricularius L. Hassk. | aerial parts | meroterpenoids with pyrone-sesquiterpenoid hybrid skeletons: pogostemins A-C | SW480, SK-LU-1 | [230] |
| Pogostemon cablin (Blanco) Benth | aerial parts | phenylethanoid glycosides: verbascoside, pedicularioside G () | A549, HCT-15 | [231] |
| Premna odorata Blanco | bark | tetrahydrofurofuran lignin: 4β-hydroxyasarinin | HT-29, MCF-7 | [232] |
| Rabdosia serra (Maxim.) Hara | grasses | diterpenoids: rabdosins E–K, serrin B, serrin A, isodocarpin and lushanrubescensin J | A549, NCI--H661 | [233] |
| Salvia ballotiflora Benth | leaves | diterpenoids: 7α-acetoxy-6,7-dihydroicetexone, anastomosine | SK-LU-1 | [234] |
| Salvia ballotiflora Benth. | aerial parts | diterpenes: 19-deoxyicetexone,7,20-dihydroanastomosine, icetexoneand19-deoxyisoicetexone, | A549, MCF-7 | [127] |
| Salvia lachnocalyx Hedge | shoots | (2Z,6Z,10Z,14E)-geranylfarnesol and spathulenol | MCF-7, HT-29 | [235] |
| Salvia leriifolia Benth. | aerial parts | labdane diterpenoids: 6β,13β-dihydroxylabd-8, 14-diene-19-oic acid, 13-hydroxylabd-8, 14-diene-6β,19-olide, 8,12E,14-labdatrien-6,19-olid | MDA-MB-231, DU 145 | [236] |
| Salvia leucantha Cav | aerial parts | neoclerodane diterpenoids: leucansalvialins FeI (1–4), and one rare 18(4→3)-abeo-abietane diterpenoid, leucansalvialin J | A549, MCF-7, SW480 | [237] |
| Salvia officinalis L. | leaves | diterpene: manool | MCF-7 | [238] |
| Salvia sahendica Boiss. & Buhs | leaves | terpenoid: sclareol | MDA-MB-231 | [239] |
| Salvia santolinifolia Boiss | callus | salvialactomine, 5-Methylflavone | PC-3 | [240] |
| Salvia tebesana Bunge | roots | diterpenoids: tebesinone A (1) and tebesinone B (2), aegyptinone A (3) andaegyptinone B (4) | MCF-7, PC- | [241] |
| Salvia tiliifolia L. | aerial parts | neo-clerodane diterpenoids: tiliifolins A–E | A549, MCF-7, SW480 | [242] |
| Salvia urmiensis Bunge | aerial parts | triterpenoids: urmiensolide B and urmiensic acid | MCF-7 | [243] |
| Scutellaria barbata D. Don | whole plant | neoclerodane diterpenoids: barbatin F, barbatin G, scutebata A, scutebata B, scutebata C and scutebata P | LoVo, MCF-7, HCT-116 | [244] |
| Scutellaria barbata D. Don | aerial parts | neo-clerodane diterpenoids: 13(R*)-1β,6α-dibenzoyloxy-7β-hydroxy-8β,13-epoxy-3-neo-cleroden-15,16-olide (scutebata C1), 2-oxo-6α-nicotinoyloxy-7β-benzoyloxy-8β-hy-droxy-3,11(E),13-neo-clerodatrien-15,16-olide.(scutebata X), 13(R*)-2-oxo-6α-acetoxy-7β-nicotinoyloxy-8β,13-epoxy-3-neo-cleroden-15,16-olide, (scutebata A1) | MCF-7, A549 | [245] |
| Scutellaria barbata D. Don | aerial parts | neoclerodane diterpenoid: barbatin H, scutebata P, scutebarbatine F, 6-O-nicotinoylscutebarbatine G, scutebata G, scutebata E, scutebata D, barbatin C, scutebarbatine A, scutebartine G, scutebarbatine B, 6,7-Di-O-acetoxybarbatin A, scutebata C, scutebata A, scutebarbatine X, scutebata B | LoVo, MCF-7, HCT-116 | [246] |
| Scutellaria barbata D.Don | whole plant | neo-clerodane diterpenoids: scutebarbatolides A-C, 4-deoxy-11,12-didehydroandrographolide, scutehenanine H, 14β-hydroxyscutolideK | LNCaP, MCF-7 | [247] |
| Scutellaria coleifolia Levl. | aerial parts | neo-clerodane type diterpenoids: scutefolides G1-S | A549, MCF-7 | [248] |
| Scutellaria strigillosa Hemsley, J. Linn. Soc | whole plant | neo-clerodane diterpenoids: scutestrigillosins A-C | MCF-7, HT-29 | [249] |
| Teucrium polium L. | aerial parts | saponin glycosides: poliusaposide A, poliusaposide B, poliusaposide C | MDA-MB-468, HCC-2998, COLO 205 | [250] |
| Teucrium ramosissimum Desf. | leaves | sesquiterpene: β-eudesmol | A549, HT-29, Caco-2 | [251] |
| Teucrium viscidum Blum. | whole plants | abietane diterpenoid: teuvisone, a pair of new dimeric abietane diterpenoid stereoisomers: biteuvisones A and B, and three sesquiterpenoid lactones, teuvislactones A−C | teuvisone and biteuvisones B showed cytotoxic effect against NCI-H460, HCT-116 | [252] |
| Thymus alternans Kloko. | aerial parts | triterpenes: 3a-hydroxy-urs-12,15-diene, a-amyrin, b-amyrin, isoursenol, epitaraxerol, and oleanolic acid | MDA-MB-231, HCT-15 HCT-116, U1810 | [253] |
| Name of The Species | Part of the Plant | Compounds Identified in Essential Oils | Cancer Cell Lines | Ref. |
|---|---|---|---|---|
| Anisomeles indica Kuntze. | leaves | The major compounds: farnesyl acetone (10.67%), nootkatone (8.35%), phytol acetate (7.35%), jasmatone (7.81%) | A549 | [284] |
| Ballota undulata, Ballota saxatilis Ballota nigra ssp. foetida | aerial parts | Ballota nigra ssp. foetida: germacrene D (23.1%), (E)-β-caryophyllene (20.3%) and caryophyllene oxide (6.2%), Ballota saxatilis: linalool (11.2%), (E)-β-caryophyllene (8.8%), caryophyllene oxide (6.3%) and (E)-2-hexenal (5.6%), Ballota undulata: germacrene D (16.0%), and bicyclogermacrene (10.4%) | MCF-7 | [285] |
| Cantinoa stricta (Benth.) Harley and J.F.B. Pastore (formely Hyptis stricta Benth.) | leaves and flowers | The major compounds: caryophyllene oxide (leaf –31.6%; flower –21.7%) and cis-pinane (leaf –15.4%; flower –9.7%), α-pinene (9.4%) and β-pinene (9.1%) | MCF-7, NCI-H460, PC-3 | [286] |
| Cedronella canariensis var. canariensis (L.) Webb and Berthel. | aerial parts | The major compounds: pinocarvone (58.0%) and β-pinene (10.8%) | MDA-MB-231, HCT-116 | [287] |
| Cunila angustifolia Benth. | leaves | The major compounds: pulegone (29.5%), isomenthol (27.0%), menthone (8.6%), neomenthol (7.2%), menthyl acetate (2.5%), and caryophyllene oxide (2.0%) | A-549, MCF-7 | [288] |
| Elsholtzia ciliata (Thunb.) Hylander | aerial parts | The major compounds: dehydroelsholtzia ketone, elsholtzia ketone, sesquiterpenes β-bourbonene, caryophyllene, α-caryophyllene, germacrene D, and α-farnesene | MDA-MB-231 | [289] |
| Lavandula hybrid Rev., Lavandula latifolia Medikus., Lavandula vera D.C. and Lavandula angustifolia Miller. | aerial parts | Terpenes: linalool and linalyl acetate terpenoids: 1,8-cineole | Caco-2 | [290] |
| Leonotis nepetifolia (L.) R.Br. | leaves | The major compounds: 3-octanone (3.75%), (E)-ocimene (15.85%), (Z)-ocimene (7.01%), linalool, caryophillene oxide, and 1-octen-3-ol (42.58%) | HCT-116 | [291] |
| Melissa officinalis L. | leaves | citral (47.2%), caryophyllene oxide (10.2%), citronellal (5.4%), geraniol (6.6%), geranyl acetate (4.1%) and β- caryophyllene (3,8%) | MCF-7, NCI-H460 | [292] |
| Mentha citrate Ehrh. | aerial part | The major compounds: linalool (34.69%), linalyl acetate (35.75%) | HCT-116 | [293] |
| Mentha spicata L. | aerial parts | The major compounds: carvone (49.5%), limonene (16.1%), 1,8-cin-eole (8.7%), cis-dihydrocarvone (3.9%), β-caryophyllene (2.7%), germacrene D (2.1%), and β-pinene (1.1%) | HCT-116, MCF-7 | [294] |
| Meriandra dianthera (Konig ex Roxb.) Benth. | aerial parts | camphor (54.3%), 1,8-cineole (12.2%), and camphene (10.4%) | MCF-7, LoVo | [295] |
| Nepeta menthoides Boiss. and Bohse. | - | The major compounds: 1,8-cineole (70.06%) | MCF-7, T-47D, MDA-MB-231 | [296] |
| Nepeta schiraziana Boiss. | aerial parts | The major compounds: 1,8-cineole (33.67%), germacrene D (11.45%), β-caryophyllene (9.88%), and caryophyllene oxide (7.34%) | MCF-7 | [297] |
| Nepeta sintenisii Bornm. | aerial parts | 4aα,7α7aβ nepetalactone (51.74%), β-farnesene (12.26%), 4aα,7α,7aα nepetalactone (8.01%), germacrene-D (5.01%), and 4aα7β,7aα-nepetalactone (3.71%) | LS180, MCF-7 | [298] |
| Ocimum basilicum L., Mentha spicata L. | chopped leaves and stems | Mentha spicata: carvone (85.4%), limonene (8.4%), and β-Pinene (1.4%) Ocimum basilicum: methyl chavicol (74.9%), linalol (18.4%) | MCF-7, Caco2 | [299] |
| Origanum majorana L. | aerial parts | The major compounds: terpinen-4-ol (23.2%), Cis-sabinene hydrate (17.5%), γ-terpinene (10.5%), p-cymene (9%), α-terpineol (5.6%), α-terpinene (4.7%), and trans-sabinene hydrate (4.0%) | HT-29 | [300] |
| Origanum dictamnus (L.) Kostel., Origanum libanoticum Boiss., and Origanum microphyllum (Bentham) T. Vogel | - | The major compounds: carvacrol, p-cymene, linalool, γ-terpinene, and terpinen-4-ol as | LoVo | [301] |
| Origanum vulgare L. | leaves | pulegone (77.4%), menthone (4.8%), cis-Isopulegone (2.2%), piperitenone (2.1%), limonene (1.0%), and myrcene (0.6%) | MCF-7, HT-29 | [302] |
| Plectranthus. cylindraceus Hocst. ex Benth., Plectranthus. asirensis JRI Wood. and Plectranthus barbatus Andrews. | leaves and branches | The major compounds: α-pinene (46.2%), maaliol (42.8%), and β-caryophyllene (13.3%) | HT-29 | [303] |
| Premna microphylla Turcz. | aerial parts | The major compounds: blumenol C (49.7%), β-cedrene (6.1%), limonene (3.8%), α-guaiene (3.3%), cryptone (3.1%), and gurkeα-cyperone (2.7%) | MCF-7 | [304] |
| Rosmarinus officinalis L. | aerial parts | The major compounds: 1,8-cineole (23.56%), camphene (12.78%), camphor (12.55%), and β-pinene (12.3%) | MCF-7 | [305] |
| Salvia macrosiphon Boiss. | aerial parts | The major compounds: linalool (19%), β-cedrene (14.64%), and β-elemene (13.33%) | MCF-7, MDA-MB-231, T-47D | [306] |
| Salvia officinalis L. | aerial parts | The major compounds: α-thujone, 1,8-cineole, and camphor | LNCaP, MCF-7 | [307] |
| Salvia officinalis L. | aerial parts | α-thujone (29.39%), 1,8-cineole (eucalyptol 22.8%), and camphor (13.05%) | Caco-2, HT-29, HCT-116 | [308] |
| Salvia ringens Sibth. and Sm. | whole plant | The major compounds: 1.8-cineole (31.99%), cam-phene (17.06%), borneol (11.94%), and α-pinene (11.52%) | HCT-116 | [309] |
| Satureja intermedia C.A.Mey | aerial parts | γ-terpinene (37.1%), thymol (30.2%), p-cymene (16.2%), limonene (3.9%), α-terpinene (3.3), myrcene (2.5%), germacrene B (1.4%), elemicine (1.1%), and carvacrol (0.5%) | MCF-7 | [310] |
| Satureja thymbra L. and Satureja parnassica Heldr. and Sart. ex Boiss | aerial parts | The major compounds: carvacrol, thymol, γ-terpinene, and p-cymene | MCF-7, A549 | [311] |
| Stachys annua L. subsp. annua | aerial parts | The major compounds: phytol (9.8%), germacrene D (9.2%), and spathulenol (8.5%) | HCT-116, MDA-MB-231 | [312] |
| Stachys annua L. subsp. annua | aerial parts | phytol (9.8%), germacrene D (9.2%), and spathulenol (8.5%) | MDA-MB 231, HCT-116 | [312] |
| Stachys parviflora L. | aerial parts | The major compounds: α-terpenylacetate (23.6%), caryophyllene (16.8%), bicyclogermacrene (9.3%), spathulenol (4.9%), and α-pinene (4.2%) | HCT-116 | [313] |
| Tetradenia riparia (Hochst.) Codd. | leaves | fenchone (6.1%), dronabinol (11.0%), aromadendrene oxide (14.7%), and (E,E)–farnesol (15.0%) | HT-29, MCF-7 | [314] |
| Thymus alternans Klokov. | aerial parts | The major compounds: (E)-nerolidol, neryl acetate, nerol | MDA-MB-231, HCT-15, HCT-116, U1810 | [253] |
| Thymus munbyanus subsp. Coloratus (Boiss. and Reut.) Greuter and Burdet | stems, leaves | borneol (44.8 and 31.2%) Other components occurring in noteworthy levels were camphor (5.7 and 13.6%), camphene (3.6 and 7.5%), 1,8-cineole (6.0 and 4.2%), and germacrene D (5.0 and 3.1%) | MDA-MB-231 | [315] |
| Zataria multiflora Boiss. | aerial parts | - | MCF-7 | [316] |
| Zataria multiflora Boiss. Satureja bakhtiarica Bunge. | leaves | The major compounds: phenol (56.35%, 37.4%), thymol (13.82%, 22.6%), P-cymene (8.79%, 19.3%), γ-terpinene (3.36%, 5.0%), β-myrcene (1.91%, 0.8%), β-caryophyllene (1.28%, 2.2%), α-terpinene (1.21%, 0.9%), caryophyllene oxide (0.47%, 2.0%), and carvacrole (2.88%, 0.2%) | MDA-MB-231 | [317] |
| Zhumeria majdae Rech.f. and Wendelbo. | aerial parts | The major compounds: linalool (24.4–34.6%), camphor (26.1–34.7%), and translinalool oxide (7.6–28.6%) | MCF-7 | [318] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitarek, P.; Merecz-Sadowska, A.; Śliwiński, T.; Zajdel, R.; Kowalczyk, T. An In Vitro Evaluation of the Molecular Mechanisms of Action of Medical Plants from the Lamiaceae Family as Effective Sources of Active Compounds against Human Cancer Cell Lines. Cancers 2020, 12, 2957. https://doi.org/10.3390/cancers12102957
Sitarek P, Merecz-Sadowska A, Śliwiński T, Zajdel R, Kowalczyk T. An In Vitro Evaluation of the Molecular Mechanisms of Action of Medical Plants from the Lamiaceae Family as Effective Sources of Active Compounds against Human Cancer Cell Lines. Cancers. 2020; 12(10):2957. https://doi.org/10.3390/cancers12102957
Chicago/Turabian StyleSitarek, Przemysław, Anna Merecz-Sadowska, Tomasz Śliwiński, Radosław Zajdel, and Tomasz Kowalczyk. 2020. "An In Vitro Evaluation of the Molecular Mechanisms of Action of Medical Plants from the Lamiaceae Family as Effective Sources of Active Compounds against Human Cancer Cell Lines" Cancers 12, no. 10: 2957. https://doi.org/10.3390/cancers12102957
APA StyleSitarek, P., Merecz-Sadowska, A., Śliwiński, T., Zajdel, R., & Kowalczyk, T. (2020). An In Vitro Evaluation of the Molecular Mechanisms of Action of Medical Plants from the Lamiaceae Family as Effective Sources of Active Compounds against Human Cancer Cell Lines. Cancers, 12(10), 2957. https://doi.org/10.3390/cancers12102957