PUMA and NOXA Expression in Tumor-Associated Benign Prostatic Epithelial Cells Are Predictive of Prostate Cancer Biochemical Recurrence
Abstract
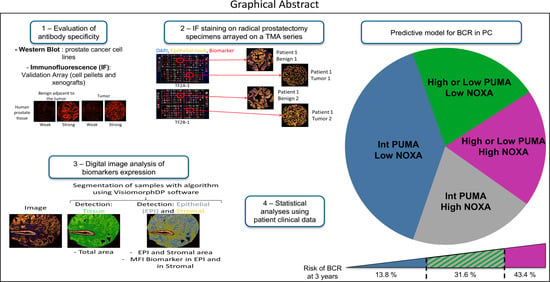
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics and Clinical Parameters
2.2. Multiplex Staining of TMA Cores
2.3. Expression of PUMA and NOXA Can Independently Predict BCR
2.4. Combining Expression Levels of PUMA and NOXA in Benign Cells Can Predict BCR
3. Discussion
4. Materials and Methods
4.1. Antibody Validation
4.2. Paraffin Processing and Embedding of Cell Line Pellets
4.3. siRNA Transfection
4.4. Western Blot Analysis
4.5. Patient Cohort
4.6. Construction of the Tissue Microarray
4.7. Immunofluorescence
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quinlan, D.M.; Partin, A.W.; Walsh, P.C. Can aggressive prostatic carcinomas be identified and can their natural history be altered by treatment? Urology 1995, 46, 77–82. [Google Scholar] [CrossRef]
- Etzioni, R.; Penson, D.F.; Legler, J.M.; di Tommaso, D.; Boer, R.; Gann, P.H.; Feuer, E.J. Overdiagnosis due to prostate-specific antigen screening: Lessons from U.S. prostate cancer incidence trends. J. Natl. Cancer Inst. 2002, 94, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.I. An update of the Gleason grading system. J. Urol. 2010, 183, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, A.M.; Kweldam, C.F.; van Leenders, G.J. Prognostic histopathological and molecular markers on prostate cancer needle-biopsies: A review. BioMed Res. Int. 2014, 2014, 341324. [Google Scholar] [CrossRef] [Green Version]
- Egevad, L.; Judge, M.; Delahunt, B.; Humphrey, P.A.; Kristiansen, G.; Oxley, J.; Rasiah, K.; Takahashi, H.; Trpkov, K.; Varma, M.; et al. Dataset for the reporting of prostate carcinoma in core needle biopsy and transurethral resection and enucleation specimens: Recommendations from the International Collaboration on Cancer Reporting (ICCR). Pathology 2019, 51, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Shibue, T.; Taniguchi, T. BH3-only proteins: Integrated control point of apoptosis. Int. J. Cancer 2006, 119, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Villunger, A.; Michalak, E.M.; Coultas, L.; Mullauer, F.; Bock, G.; Ausserlechner, M.J.; Adams, J.M.; Strasser, A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003, 302, 1036–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamzami, N.; Kroemer, G. p53 in apoptosis control: An introduction. Biochem. Biophys. Res. Commun. 2005, 331, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Hikisz, P.; Kilianska, Z.M. PUMA, a critical mediator of cell death—One decade on from its discovery. Cell Mol. Biol. Lett. 2012, 17, 646–669. [Google Scholar] [CrossRef]
- Chen, L.; Willis, S.N.; Wei, A.; Smith, B.J.; Fletcher, J.I.; Hinds, M.G.; Colman, P.M.; Day, C.L.; Adams, J.M.; Huang, D.C. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 2005, 17, 393–403. [Google Scholar] [CrossRef]
- Westphal, D.; Dewson, G.; Czabotar, P.E.; Kluck, R.M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta 2011, 1813, 521–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.L.; Yao, D.B.; Zhao, Y.; Xu, F.; Jia, C.J.; Xu, Y.Q.; Dai, C.L. Prognostic value of PUMA expression in patients with HBV-related hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 38–44. [Google Scholar]
- Ahn, C.H.; Jeong, E.G.; Kim, S.S.; Lee, J.W.; Lee, S.H.; Kim, S.H.; Kim, M.S.; Yoo, N.J.; Lee, S.H. Expressional and mutational analysis of pro-apoptotic Bcl-2 member PUMA in hepatocellular carcinomas. Dig. Dis. Sci. 2008, 53, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.S.; Lv, F.; Yang, Z.L.; Miao, X.Y. Immunohistochemical study of PUMA, c-Myb and p53 expression in the benign and malignant lesions of gallbladder and their clinicopathological significances. Int. J. Clin. Oncol. 2013, 18, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Li, Q.; Yang, Z.; Miao, X.; Wen, Y.; Huang, S.; Ouyang, J. Expression of p53 upregulated modulator of apoptosis (PUMA) and C-myb in gallbladder adenocarcinoma and their pathological significance. Clin. Transl. Oncol. 2013, 15, 818–824. [Google Scholar] [CrossRef]
- Diallo, J.S.; Aldejmah, A.; Mouhim, A.F.; Peant, B.; Fahmy, M.A.; Koumakpayi, I.H.; Sircar, K.; Begin, L.R.; Mes-Masson, A.M.; Saad, F. NOXA and PUMA expression add to clinical markers in predicting biochemical recurrence of prostate cancer patients in a survival tree model. Clin. Cancer Res. 2007, 13, 7044–7052. [Google Scholar] [CrossRef] [Green Version]
- Labouba, I.; Le Page, C.; Communal, L.; Kristessen, T.; You, X.; Peant, B.; Barres, V.; Gannon, P.O.; Mes-Masson, A.M.; Saad, F. Potential Cross-Talk between Alternative and Classical NF-kappaB Pathways in Prostate Cancer Tissues as Measured by a Multi-Staining Immunofluorescence Co-Localization Assay. PLoS ONE 2015, 10, e0131024. [Google Scholar] [CrossRef]
- Zhou, X.; Li, J.; Marx, C.; Tolstov, Y.; Rauch, G.; Herpel, E.; Macher-Goeppinger, S.; Roth, W.; Grullich, C.; Pahernik, S.; et al. Uncoupling of PUMA Expression and Apoptosis Contributes to Functional Heterogeneity in Renal Cell Carcinoma—Prognostic and Translational Implications. Transl. Oncol. 2015, 8, 480–486. [Google Scholar] [CrossRef] [Green Version]
- Sakakibara-Konishi, J.; Oizumi, S.; Kikuchi, J.; Kikuchi, E.; Mizugaki, H.; Kinoshita, I.; Dosaka-Akita, H.; Nishimura, M. Expression of Bim, Noxa, and Puma in non-small cell lung cancer. BMC Cancer 2012, 12, 286. [Google Scholar] [CrossRef]
- Zhu, H.J.; Liu, L.; Fan, L.; Zhang, L.N.; Fang, C.; Zou, Z.J.; Li, J.Y.; Xu, W. The BH3-only protein Puma plays an essential role in p53-mediated apoptosis of chronic lymphocytic leukemia cells. Leuk. Lymphoma 2013, 54, 2712–2719. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A.; Rego, R.L.; Okumura, K.; Foster, N.R.; O’Connell, M.J.; Sargent, D.J.; Windschitl, H.E. Prognostic impact of bim, puma, and noxa expression in human colon carcinomas. Clin. Cancer Res. 2008, 14, 5810–5818. [Google Scholar] [CrossRef] [Green Version]
- Jansson, A.K.; Emterling, A.M.; Arbman, G.; Sun, X.F. Noxa in colorectal cancer: A study on DNA, mRNA and protein expression. Oncogene 2003, 22, 4675–4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergstrom, S.H.; Jaremo, H.; Nilsson, M.; Adamo, H.H.; Bergh, A. Prostate tumors downregulate microseminoprotein-beta (MSMB) in the surrounding benign prostate epithelium and this response is associated with tumor aggressiveness. Prostate 2018, 78, 257–265. [Google Scholar] [CrossRef]
- Yang, B.; Etheridge, T.; McCormick, J.; Schultz, A.; Khemees, T.A.; Damaschke, N.; Leverson, G.; Woo, K.; Sonn, G.A.; Klein, E.A.; et al. Validation of an epigenetic field of susceptibility to detect significant prostate cancer from non-tumor biopsies. Clin. Epigenet. 2019, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Adamo, H.H.; Stromvall, K.; Nilsson, M.; Halin Bergstrom, S.; Bergh, A. Adaptive (TINT) Changes in the Tumor Bearing Organ Are Related to Prostate Tumor Size and Aggressiveness. PLoS ONE 2015, 10, e0141601. [Google Scholar] [CrossRef]
- Zietarska, M.; Madore, J.; Diallo, J.S.; Delvoye, N.; Saad, F.; Provencher, D.; Mes-Masson, A.M. A novel method of cell embedding for tissue microarrays. Histopathology 2010, 57, 323–329. [Google Scholar] [CrossRef]
- Gaudreau, P.O.; Clairefond, S.; Class, C.A.; Boulay, P.L.; Chrobak, P.; Allard, B.; Azzi, F.; Pommey, S.; Do, K.A.; Saad, F.; et al. WISP1 is associated to advanced disease, EMT and an inflamed tumor microenvironment in multiple solid tumors. Oncoimmunology 2019, 8, e1581545. [Google Scholar] [CrossRef] [Green Version]



| Clinical Parameters | TF123 TMA Series |
|---|---|
| Median age at RP, years (IQR) | 63 (59–67) |
| Median PSA at diagnosis, ng/mL (IQR) | 7.0 (5.0–10.8) |
| Pathological TNM | |
| 2 | 201 |
| 3 | 75 |
| 4 | 9 |
| Gleason score at RP | |
| ≤3 + 3 | 140 |
| 3 + 4 | 93 |
| 4 + 3 | 19 |
| ≥4 + 4 | 29 |
| Unknown | 4 |
| Positive margin | 95 |
| Median follow-up, months (IQR) | 129 (76–174) |
| Biochemical recurrence at 10 years | |
| No | 177 |
| Yes | 108 |
| Median time to BCR, months (IQR) | 16 (4–41) |
| Biochemical recurrence at 3 years | |
| No | 207 |
| Yes | 78 |
| Median time to BCR, months (IQR) | 8 (2–19) |
| Clinical Parameters | Univariate | Multivariate with PUMA Expression | Multivariate with NOXA Expression | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||||
| Age at Dx | 1.015 | (0.974–1.057) | 0.482 | − | − | − | − | − | − |
| PSA at Dx | 1.048 | (1.017–1.081) | 0.003 | 1.014 | (0.975–1.056) | 0.483 | 1.029 | (0.989–1.070) | 0.160 |
| Gleason score | 1.809 | (1.473–2.221) | 0.000 | 1.547 | (1.234–1.940) | 0.000 | 1.552 | (1.252–1.923) | 0.000 |
| Margin | 3.890 | (2.452–6.170) | 0.000 | 2.567 | (1.556–4.234) | 0.000 | 2.458 | (1.475–4.096) | 0.001 |
| cTNM (category) | 1.031 | (0.587–1.810) | 0.916 | − | − | − | − | − | − |
| pTNM (category) | 2.934 | (2.122–4.056) | 0.000 | 1.957 | (1.286–2.978) | 0.002 | 1.672 | (1.111–2.516) | 0.014 |
| PUMA (dichotomized) | 1.953 | (1.241–3.075) | 0.004 | 2.173 | (1.366–3.456) | 0.001 | − | − | − |
| NOXA (dichotomized) | 2.377 | (1.504–3.759) | 0.000 | − | − | − | 2.280 | (1.417–3.670) | 0.001 |
| Clinical Parameters | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |||
| Age at Dx | 1.015 | (0.974–1.057) | 0.482 | − | − | − |
| PSA at Dx | 1.048 | (1.017–1.081) | 0.003 | 1.022 | (0.981–1.065) | 0.293 |
| Gleason score (category) | 1.809 | (1.473–2.221) | 0.000 | 1.547 | (1.240–1.931) | 0.000 |
| Margin | 3.890 | (2.452–6.170) | 0.000 | 2.481 | (1.484–4.148) | 0.001 |
| cTNM (category) | 1.031 | (0.587–1.810) | 0.916 | − | − | − |
| pTNM (category) | 2.934 | (2.122–4.056) | 0.000 | 1.679 | (1.109–2.543) | 0.014 |
| Combination of PUMA_ (dichotomized) and NOXA (dichotomized) | 3.055 | (1.732–5.386) | 0.000 | 2.935 | (1.645–5.236) | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clairefond, S.; Péant, B.; Ouellet, V.; Barrès, V.; Tian, Z.; Trudel, D.; Karakiewicz, P.I.; Mes-Masson, A.-M.; Saad, F. PUMA and NOXA Expression in Tumor-Associated Benign Prostatic Epithelial Cells Are Predictive of Prostate Cancer Biochemical Recurrence. Cancers 2020, 12, 3187. https://doi.org/10.3390/cancers12113187
Clairefond S, Péant B, Ouellet V, Barrès V, Tian Z, Trudel D, Karakiewicz PI, Mes-Masson A-M, Saad F. PUMA and NOXA Expression in Tumor-Associated Benign Prostatic Epithelial Cells Are Predictive of Prostate Cancer Biochemical Recurrence. Cancers. 2020; 12(11):3187. https://doi.org/10.3390/cancers12113187
Chicago/Turabian StyleClairefond, Sylvie, Benjamin Péant, Véronique Ouellet, Véronique Barrès, Zhe Tian, Dominique Trudel, Pierre I. Karakiewicz, Anne-Marie Mes-Masson, and Fred Saad. 2020. "PUMA and NOXA Expression in Tumor-Associated Benign Prostatic Epithelial Cells Are Predictive of Prostate Cancer Biochemical Recurrence" Cancers 12, no. 11: 3187. https://doi.org/10.3390/cancers12113187
APA StyleClairefond, S., Péant, B., Ouellet, V., Barrès, V., Tian, Z., Trudel, D., Karakiewicz, P. I., Mes-Masson, A. -M., & Saad, F. (2020). PUMA and NOXA Expression in Tumor-Associated Benign Prostatic Epithelial Cells Are Predictive of Prostate Cancer Biochemical Recurrence. Cancers, 12(11), 3187. https://doi.org/10.3390/cancers12113187