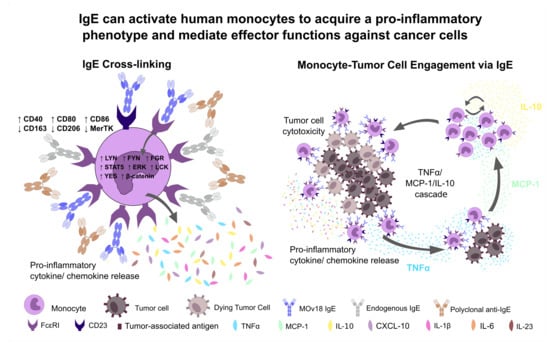
IgE Activates Monocytes from Cancer Patients to Acquire a Pro-Inflammatory Phenotype
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Cross-Linking of IgE on Human Monocytes Induces Pro-Inflammatory Cell Surface Receptor Expression Profiles
2.2. Cross-Linking of FcεR-Bound IgE Increases Production of Pro-Inflammatory Mediators by Monocytes
2.3. Cross-Linking IgE on the Monocyte Surface Activates Protein Kinases Downstream of the FcεRI and Activation of Several Immune Pathways
2.4. IgE Potentiates Cytotoxic Killing of Tumour Cells and Immune Activatory Functions of Monocytes from Healthy Volunteers and Ovarian Cancer Patients
2.5. IgE-Stimulated Monocytes Can Secrete TNFα, MCP-1 and IL-10
2.6. IgE-Mediated Immune Mediator Signatures Are Associated with Favorable Overall Survival in Ovarian Cancer
3. Discussion
4. Materials and Methods
4.1. Human Samples and Ethics
4.2. Primary Monocyte Stimulation by IgE Cross-Linking
4.3. Analysis of Phosphorylation Profile of Protein Kinases
4.4. Study of FcεRI Pathway and Implicated Pathways Based on Protein Kinase Analysis
4.5. Statistical Methods and Survival Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ikeda, N.; Asano, K.; Kikuchi, K.; Uchida, Y.; Ikegami, H.; Takagi, R.; Yotsumoto, S.; Shibuya, T.; Makino-Okamura, C.; Fukuyama, H.; et al. Emergence of immunoregulatory Ym1+Ly6Chi monocytes during recovery phase of tissue injury. Sci. Immunol. 2018, 3, eaat0207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguíluz-Gracia, I.; Bosco, A.; Dollner, R.; Melum, G.R.; Lexberg, M.H.; Jones, A.C.; Dheyauldeen, S.A.; Holt, P.G.; Bækkevold, E.S.; Jahnsen, F.L. Rapid recruitment of CD14 + monocytes in experimentally induced allergic rhinitis in human subjects. J. Allergy Clin. Immunol. 2016, 137, 1872–1881.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balboa, L.; Barrios-Payan, J.; González-Domínguez, É.; Lastrucci, C.; Lugo-Villarino, G.; Mata-Espinoza, D.; Schierloh, P.; Kviatcovsky, D.; Neyrolles, O.; Maridonneau-Parini, I.; et al. Diverging biological roles among human monocyte subsets in the context of tuberculosis infection. Clin. Sci. 2015, 129, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Carter, R.A.; Leiner, I.M.; Tang, Y.-W.; Chen, L.; Kreiswirth, B.N.; Pamer, E.G. Distinct Contributions of Neutrophils and CCR2+Monocytes to Pulmonary Clearance of Different Klebsiella pneumoniae Strains. Infect. Immun. 2015, 83, 3418–3427. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Bruder, D.; Wolf, S.A.; Jeron, A.; Mack, M.; Heimesaat, M.M.; Dunay, I.R. Ly6Chigh Monocytes Control Cerebral Toxoplasmosis. J. Immunol. 2015, 194, 3223–3235. [Google Scholar] [CrossRef] [Green Version]
- Canè, S.; Ugel, S.; Trovato, R.; Marigo, I.; De Sanctis, F.; Sartoris, S.; Bronte, V. The Endless Saga of Monocyte Diversity. Front. Immunol. 2019, 10, 1786. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, M.; Fischer, H.; Schackert, G. Activated monocytes kill malignant brain tumor cellsin vitro. J. Neuro-Oncol. 1994, 20, 35–45. [Google Scholar] [CrossRef]
- Karagiannis, S.N.; Bracher, M.G.; Hunt, J.; McCloskey, N.; Beavil, R.L.; Beavil, A.J.; Fear, D.J.; Thompson, R.G.; East, N.; Burke, F.; et al. IgE-Antibody-Dependent Immunotherapy of Solid Tumors: Cytotoxic and Phagocytic Mechanisms of Eradication of Ovarian Cancer Cells. J. Immunol. 2007, 179, 2832–2843. [Google Scholar] [CrossRef] [Green Version]
- Karagiannis, S.N.; Josephs, D.H.; Bax, H.J.; Spicer, J.F. Therapeutic IgE Antibodies: Harnessing a Macrophage-Mediated Immune Surveillance Mechanism against Cancer. Cancer Res. 2017, 77, 2779–2783. [Google Scholar] [CrossRef] [Green Version]
- Spicer, J.; Basu, B.; Montes, A.; Banerji, U.; Kristeleit, R.; Veal, G.J.; Corrigan, C.; Till, S.; Nintos, G.; Brier, T.; et al. Abstract CT141: Phase 1 trial of MOv18, a first-in-class IgE antibody therapy for cancer. Cancer Res. 2020, 80. [Google Scholar] [CrossRef]
- Kinet, J.-P. THE HIGH-AFFINITY IgE RECEPTOR (FcεRI): From Physiology to Pathology. Annu. Rev. Immunol. 1999, 17, 931–972. [Google Scholar] [CrossRef]
- Josephs, D.H.; Bax, H.J.; Dodev, T.; Georgouli, M.; Nakamura, M.; Pellizzari, G.; Saul, L.; Karagiannis, P.; Cheung, A.; Herraiz, C.; et al. Anti-Folate Receptor-α IgE but not IgG Recruits Macrophages to Attack Tumors via TNFα/MCP-1 Signaling. Cancer Res. 2017, 77, 1127–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josephs, D.H.; Bax, H.J.; Karagiannis, S.N. Tumour-associated macrophage polarisation and re-education with immunotherapy. Front. Biosci. 2015, 7, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Josephs, D.H.; Bax, H.J.; Lentfer, H.; Selkirk, C.; Spicer, J.F.; Karagiannis, S.N. Potential for monocyte recruitment by IgE immunotherapy for cancer in a rat model of tumour metastasis. Lancet 2015, 385, S53. [Google Scholar] [CrossRef] [Green Version]
- Karagiannis, S.N.; Wang, Q.; East, N.; Burke, F.; Riffard, S.; Bracher, M.G.; Thompson, R.G.; Durham, S.R.; Schwartz, L.B.; Balkwill, F.; et al. Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells. Eur. J. Immunol. 2003, 33, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Starkl, P.; Marichal, T.; Galli, S.J. IgE and mast cells in host defense against parasites and venoms. Semin. Immunopathol. 2016, 38, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Platzer, B.; Elpek, K.G.; Cremasco, V.; Baker, K.; Stout, M.M.; Schultz, C.; Dehlink, E.; Shade, K.T.; Anthony, R.M.; Blumberg, R.S.; et al. IgE/FcepsilonRI-Mediated Antigen Cross-Presentation by Dendritic Cells Enhances Anti-Tumor Immune Responses. Cell Rep. 2015, 10, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Singhal, S.; Stadanlick, J.E.; Annunziata, M.J.; Rao, A.S.; Bhojnagarwala, P.S.; O’Brien, S.; Moon, E.K.; Cantu, E.; Danet-Desnoyers, G.; Ra, H.-J.; et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci. Transl. Med. 2019, 11, eaat1500. [Google Scholar] [CrossRef]
- Maurer, D.; Fiebiger, E.; Reininger, B.; Wolff-Winiski, B.; Jouvin, M.H.; Kilgus, O.; Kinet, J.P.; Stingl, G. Expression of functional high affinity immunoglobulin E receptors (Fc epsilon RI) on monocytes of atopic individuals. J. Exp. Med. 1994, 179, 745–750. [Google Scholar] [CrossRef]
- Sutton, B.J.; Davies, A.M. Structure and dynamics of IgE-receptor interactions: Fc?RI and CD23/Fc?RII. Immunol. Rev. 2015, 268, 222–235. [Google Scholar] [CrossRef]
- Pellizzari, G.; Bax, H.J.; Josephs, D.H.; Gotovina, J.; Jensen-Jarolim, E.; Spicer, J.F.; Karagiannis, S.N. Harnessing Therapeutic IgE Antibodies to Re-educate Macrophages against Cancer. Trends Mol. Med. 2020, 26, 615–626. [Google Scholar] [CrossRef]
- Pellizzari, G.; Hoskin, C.; Crescioli, S.; Mele, S.; Gotovina, J.; Chiaruttini, G.; Bianchini, R.; Ilieva, K.; Bax, H.J.; Papa, S.; et al. IgE re-programs alternatively-activated human macrophages towards pro-inflammatory anti-tumoural states. EBioMedicine 2019, 43, 67–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josephs, D.H.; Nakamura, M.; Bax, H.J.; Dodev, T.S.; Muirhead, G.; Saul, L.; Karagiannis, P.; Ilieva, K.M.; Crescioli, S.; Gazinska, P.; et al. An immunologically relevant rodent model demonstrates safety of therapy using a tumour-specific IgE. Allergy 2018, 73, 2328–2341. [Google Scholar] [CrossRef] [PubMed]
- Bracher, M.; Gould, H.J.; Sutton, B.J.; Dombrowicz, D.; Karagiannis, S.N. Three-colour flow cytometric method to measure antibody-dependent tumour cell killing by cytotoxicity and phagocytosis. J. Immunol. Methods 2007, 323, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Vouldoukis, I.; Mazier, D.; Moynet, D.; Thiolat, D.; Malvy, D.; Mossalayi, D. IgE Mediates Killing of Intracellular Toxoplasma gondii by Human Macrophages through CD23-Dependent, Interleukin-10 Sensitive Pathway. PLoS ONE 2011, 6, e18289. [Google Scholar] [CrossRef] [Green Version]
- Kalesnikoff, J.; Huber, M.; Lam, V.; E Damen, J.; Zhang, J.; Siraganian, R.P.; Krystal, G. Monomeric IgE Stimulates Signaling Pathways in Mast Cells that Lead to Cytokine Production and Cell Survival. Immunity 2001, 14, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Siraganian, R.P. Mast cell signal transduction from the high-affinity IgE receptor. Curr. Opin. Immunol. 2003, 15, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Adamczewski, M.; Numerof, R.P.; A Koretzky, G.; Kinet, J.P. Regulation by CD45 of the tyrosine phosphorylation of high affinity IgE receptor beta- and gamma-chains. J. Immunol. 1995, 154, 3047–3055. [Google Scholar]
- Razin, E.; Rivera, J. Signal Transduction in Mast Cells and Basophils; Springer: New York, NY, USA, 1999; Volume 1, pp. 102–104. [Google Scholar]
- Eiseman, E.; Bolen, J.B. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nat. Cell Biol. 1992, 355, 78–80. [Google Scholar] [CrossRef]
- Takeda, K.; Tanaka, T.; Shi, W.; Matsumoto, M.; Minami, M.; Kashiwamura, S.-I.; Nakanishi, K.; Yoshida, N.; Kishimoto, T.; Akira, S. Essential role of Stat6 in IL-4 signalling. Nat. Cell Biol. 1996, 380, 627–630. [Google Scholar] [CrossRef]
- Swartz, E.; Matsumura, S.; Han, Y.; Gibbons, A.; Hsieh, F. Il-4 Induces The Expression of The Low Affinity IgE Receptor (CD23) on Human Mast Cells. J. Allergy Clin. Immunol. 2008, 121, S15. [Google Scholar] [CrossRef]
- Defrance, T.; Aubry, J.P.; Rousset, F.; Vanbervliet, B.; Bonnefoy, J.Y.; Arai, N.; Takebe, Y.; Yokota, T.; Lee, F.; Arai, K. Human recombinant interleukin 4 induces Fc epsilon receptors (CD23) on normal human B lymphocytes. J. Exp. Med. 1987, 165, 1459–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, H.J.; Mackay, G.A.; Karagiannis, S.N.; O’Toole, C.M.; Marsh, P.J.; Daniel, B.E.; Coney, L.R.; Zurawski, V.R.; Joseph, M.; Capron, M.; et al. Comparison of IgE and IgG antibody-dependent cytotoxicity in vitro and in a SCID mouse xenograft model of ovarian carcinoma. Eur. J. Immunol. 1999, 29, 3527–3537. [Google Scholar] [CrossRef]
- Karagiannis, S.N.; Josephs, D.H.; Karagiannis, P.; Gilbert, A.E.; Saul, L.; Rudman, S.M.; Dodev, T.; Koers, A.; Blower, P.J.; Corrigan, C.; et al. Recombinant IgE antibodies for passive immunotherapy of solid tumours: From concept towards clinical application. Cancer Immunol. Immunother. 2012, 61, 1547–1564. [Google Scholar] [CrossRef] [PubMed]
- Long, K.B.; Gladney, W.L.; Tooker, G.M.; Graham, K.; Fraietta, J.A.; Beatty, G.L. IFN and CCL2 Cooperate to Redirect Tumor-Infiltrating Monocytes to Degrade Fibrosis and Enhance Chemotherapy Efficacy in Pancreatic Carcinoma. Cancer Discov. 2016, 6, 400–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef]
- Kuhn, S.; Yang, J.; Ronchese, F. Monocyte-Derived Dendritic Cells Are Essential for CD8+ T Cell Activation and Antitumor Responses After Local Immunotherapy. Front. Immunol. 2015, 6, 584. [Google Scholar] [CrossRef] [Green Version]
- Platzer, B.; Baker, K.; Vera, M.P.; Singer, K.; Panduro, M.; Lexmond, W.S.; Turner, D.; Vargas, S.O.; Kinet, J.-P.; Maurer, D.; et al. Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites. Mucosal Immunol. 2015, 8, 516–532. [Google Scholar] [CrossRef] [Green Version]
- Eplatzer, B.; Stout, M.; Fiebiger, E. Functions of dendritic-cell-bound IgE in allergy. Mol. Immunol. 2015, 68, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Etzerodt, A.; Tsalkitzi, K.; Maniecki, M.; Damsky, W.; Delfini, M.; Baudoin, E.; Moulin, M.; Bosenberg, M.; Graversen, J.H.; Auphan-Anezin, N.; et al. Specific targeting of CD163+ TAMs mobilizes inflammatory monocytes and promotes T cell–mediated tumor regression. J. Exp. Med. 2019, 216, 2394–2411. [Google Scholar] [CrossRef]
- Haque, A.R.; Moriyama, M.; Kubota, K.; Ishiguro, N.; Sakamoto, M.; Chinju, A.; Mochizuki, K.; Sakamoto, T.; Kaneko, N.; Munemura, R.; et al. CD206+ tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Zhou, Y.; Fei, M.; Zhang, G.; Liang, W.-C.; Lin, W.; Wu, Y.; Piskol, R.; Ridgway, J.; McNamara, E.; Huang, H.; et al. Blockade of the Phagocytic Receptor MerTK on Tumor-Associated Macrophages Enhances P2X7R-Dependent STING Activation by Tumor-Derived cGAMP. Immunity 2020, 52, 357–373.e9. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Bax, H.J.; Scotto, D.; Souri, E.A.; Sollie, S.; Harris, R.J.; Hammar, N.; Walldius, G.; Winship, A.; Ghosh, S.; et al. Immune mediator expression signatures are associated with improved outcome in ovarian carcinoma. OncoImmunology 2019, 8, e1593811. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, K.M.; Fazekas-Singer, J.; Bax, H.J.; Crescioli, S.; Montero-Morales, L.; Mele, S.; Sow, H.S.; Stavraka, C.; Josephs, D.H.; Spicer, J.F.; et al. AllergoOncology: Expression platform development and functional profiling of an anti-HER2 IgE antibody. Allergy 2019, 74, 1985–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bax, H.J.; Khiabany, A.; Stavraka, C.; Pellizzari, G.; Hak, C.C.W.; Robinson, A.; Ilieva, K.M.; Woodman, N.; Naceur-Lombardelli, C.; Gillett, C.; et al. Basophil activation test in cancer patient blood evaluating potential hypersensitivity to an anti-tumor IgE therapeutic candidate. Allergy 2020, 75, 2069–2073. [Google Scholar] [CrossRef]
- Mahajan, A.; Youssef, L.A.; Cleyrat, C.; Grattan, R.; Lucero, S.R.; Mattison, C.P.; Erasmus, M.F.; Jacobson, B.; Tapia, L.; Hlavacek, W.S.; et al. Allergen Valency, Dose, and FcεRI Occupancy Set Thresholds for Secretory Responses to Pen a 1 and Motivate Design of Hypoallergens. J. Immunol. 2016, 198, 1034–1046. [Google Scholar] [CrossRef] [Green Version]
- Ferastraoaru, D.; Bax, H.J.; Bergmann, C.; Capron, M.; Castells, M.; Dombrowicz, D.; Fiebiger, E.; Gould, H.J.; Hartmann, K.; Jappe, U.; et al. AllergoOncology: Ultra-Low IgE, a potential novel biomarker in cancer—A Position Paper of the European Academy of Allergy and Clinical Immunology (EAACI). Clin. Transl. Allergy 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Ferastraoaru, D.; Rosenstreich, D. IgE deficiency is associated with high rates of new malignancies: Results of a longitudinal cohort study. J. Allergy Clin. Immunol. Pract. 2020, 8, 413–415. [Google Scholar] [CrossRef]
- Shin, J.-S.; Greer, A.M. The role of FcεRI expressed in dendritic cells and monocytes. Cell. Mol. Life Sci. 2015, 72, 2349–2360. [Google Scholar] [CrossRef] [Green Version]
- Pribluda, V.S.; Pribluda, C.; Metzger, H. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc. Natl. Acad. Sci. USA 1994, 91, 11246–11250. [Google Scholar] [CrossRef] [Green Version]
- Jouvin, M.H.; Adamczewski, M.; Numerof, R.; Letourneur, O.; Vallé, A.; Kinet, J.P. Differential control of the tyrosine kinases Lyn and Syk by the two signaling chains of the high affinity immunoglobulin E receptor. J. Biol. Chem. 1994, 269, 5918–5925. [Google Scholar]
- Turner, H.; Kinet, J.-P. Signalling through the high-affinity IgE receptor FcεRI. Nat. Cell Biol. 1999, 402, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Shiue, L.; Green, J.; Green, O.M.; Karas, J.L.; Morgenstern, J.P.; Ram, M.K.; Taylor, M.K.; Zoller, M.J.; Zydowsky, L.D.; Bolen, J.B. Interaction of p72syk with the gamma and beta subunits of the high-affinity receptor for immunoglobulin E, Fc epsilon RI. Mol. Cell. Biol. 1995, 15, 272–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, T.; Kihara, H.; Bhattacharyya, S.; Sakamoto, H.; Appella, E.; Siraganian, R.P. Downstream Signaling Molecules Bind to Different Phosphorylated Immunoreceptor Tyrosine-based Activation Motif (ITAM) Peptides of the High Affinity IgE Receptor. J. Biol. Chem. 1996, 271, 27962–27968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Kurosaki, T.; Corey, S.J. Engagement of the B-cell antigen receptor activates STAT through Lyn in a Jak-independent pathway. Oncogene 2006, 26, 2851–2859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pullen, N.A.; Barnstein, B.O.; Falanga, Y.T.; Wang, Z.; Suzuki, R.; Tamang, T.D.L.; Khurana, M.C.; Harry, E.A.; Draber, P.; Bunting, K.D.; et al. Novel Mechanism for FcϵRI-mediated Signal Transducer and Activator of Transcription 5 (STAT5) Tyrosine Phosphorylation and the Selective Influence of STAT5B over Mast Cell Cytokine Production. J. Biol. Chem. 2011, 287, 2045–2054. [Google Scholar] [CrossRef] [Green Version]
- Pullen, N.A.; Falanga, Y.T.; Morales, J.K.; Ryan, J.J. The Fyn-STAT5 Pathway: A New Frontier in IgE- and IgG-Mediated Mast Cell Signaling. Front. Immunol. 2012, 3, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M.; Sato, Y.; Furumichi, M.; Morishima, K.; Tanabe, M. Fc Epsilon RI Signaling Pathway-Homo Sapiens (Human). Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa04664 (accessed on 30 April 2020).
- Daëron, M. Signaling Shifts in Allergy Responses. Science 2014, 343, 982–983. [Google Scholar] [CrossRef]
- Dema, B.; Suzuki, R.; Rivera, J. Rethinking the Role of Immunoglobulin E and Its High-Affinity Receptor: New Insights into Allergy and Beyond. Int. Arch. Allergy Immunol. 2014, 164, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Győrffy, B.; Lánczky, A.; Szállási, Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr.-Relat. Cancer 2012, 19, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Pyle, D.M.; Yang, V.S.; Gruchalla, R.S.; Farrar, J.D.; Gill, M.A. IgE cross-linking critically impairs human monocyte function by blocking phagocytosis. J. Allergy Clin. Immunol. 2013, 131, 491–500.e5. [Google Scholar] [CrossRef] [Green Version]
- Murao, K.; Imachi, H.; Momoi, A.; Sayo, Y.; Hosokawa, H.; Sato, M.; Ishida, T.; Takahara, J. Thiazolidinedione inhibits the production of monocyte chemoattractant protein-1 in cytokine-treated human vascular endothelial cells. FEBS Lett. 1999, 454, 27–30. [Google Scholar] [CrossRef]
- Murao, K.; Ohyama, T.; Imachi, H.; Ishida, T.; Cao, W.M.; Namihira, H.; Sato, M.; Wong, N.C.; Takahara, J. TNF-α Stimulation of MCP-1 Expression Is Mediated by the Akt/PKB Signal Transduction Pathway in Vascular Endothelial Cells. Biochem. Biophys. Res. Commun. 2000, 276, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Staples, K.J.; Smallie, T.; Williams, L.M.; Foey, A.; Burke, B.; Foxwell, B.M.J.; Ziegler-Heitbrock, L. IL-10 induces IL-10 in primary human monocyte-derived macrophages via the transcription factor Stat3. J. Immunol. 2007, 178, 4779–4785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; He, Q.-Y. ReactomePA: An R/Bioconductor package for reactome pathway analysis and visualization. Mol. BioSyst. 2016, 12, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Boyle, E.I.; Weng, S.; Gollub, J.; Jin, H.; Botstein, D.; Cherry, J.M.; Sherlock, G. GO: TermFinder–open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics 2004, 20, 3710–3715. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, M.; Souri, E.A.; Osborn, G.; Laddach, R.; Chauhan, J.; Stavraka, C.; Lombardi, S.; Black, A.; Khiabany, A.; Khair, D.O.; et al. IgE Activates Monocytes from Cancer Patients to Acquire a Pro-Inflammatory Phenotype. Cancers 2020, 12, 3376. https://doi.org/10.3390/cancers12113376
Nakamura M, Souri EA, Osborn G, Laddach R, Chauhan J, Stavraka C, Lombardi S, Black A, Khiabany A, Khair DO, et al. IgE Activates Monocytes from Cancer Patients to Acquire a Pro-Inflammatory Phenotype. Cancers. 2020; 12(11):3376. https://doi.org/10.3390/cancers12113376
Chicago/Turabian StyleNakamura, Mano, Elmira Amiri Souri, Gabriel Osborn, Roman Laddach, Jitesh Chauhan, Chara Stavraka, Sara Lombardi, Anna Black, Atousa Khiabany, Duaa O. Khair, and et al. 2020. "IgE Activates Monocytes from Cancer Patients to Acquire a Pro-Inflammatory Phenotype" Cancers 12, no. 11: 3376. https://doi.org/10.3390/cancers12113376
APA StyleNakamura, M., Souri, E. A., Osborn, G., Laddach, R., Chauhan, J., Stavraka, C., Lombardi, S., Black, A., Khiabany, A., Khair, D. O., Figini, M., Winship, A., Ghosh, S., Montes, A., Spicer, J. F., Bax, H. J., Josephs, D. H., Lacy, K. E., Tsoka, S., & Karagiannis, S. N. (2020). IgE Activates Monocytes from Cancer Patients to Acquire a Pro-Inflammatory Phenotype. Cancers, 12(11), 3376. https://doi.org/10.3390/cancers12113376