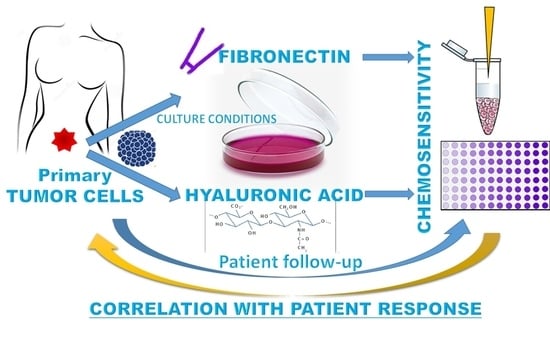
The Extracellular Matrix Influences Ovarian Carcinoma Cells’ Sensitivity to Cisplatinum: A First Step towards Personalized Medicine
Abstract
:1. Introduction
2. Results
2.1. Hyaluronic Acid and Fibronectin Were Present in the Ovarian Carcinoma Stroma and Could Modulate Tumor Cell Behavior In Vitro in Response to Chemotherapeutic Treatment
2.2. FN Was Able to Increase Cell Proliferation through MAPK Activation
2.3. FN’s Role in Regulating DNA Damage and Inducing Apoptosis in Platinum-Sensitive Cells
2.4. Adhesion and Spheroid Formation in Culture
2.5. Primary Cells Isolated from Peritoneal Fluids of Ovarian Carcinoma Patients Presented Peculiar Characteristics and Culture Behavior
2.6. Sensitivity to Cisplatinum Treatment Was Modified by the Different Matrix Cultures
2.7. Ovarian Cancer Cells’ Culture Conditions Modified the Relationship between Cisplatinum-Induced Cell Mortality and the Patient Response to Chemotherapy
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Patients
4.3. Immunohistochemical Analysis
4.4. Alcian Blue Staining
4.5. Coating Conditions
4.6. Cell Isolation and Culture
4.7. Immunofluorescence Microscopy of Ovarian Cancer Adherent Cells and Spheroids
4.8. Adhesion Assay
4.9. Proliferation Assay
4.10. Flow Cytometry
4.11. Real-Time Quantitative PCR
4.12. Pathway Analysis (PathScan® Intracellular Signaling Array Kit)
4.13. Killing Assay
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kipps, E.; Tan, D.S.; Kaye, S.B. Meeting the challenge of ascites in ovarian cancer: New avenues for therapy and research. Nat. Rev. Cancer 2013, 13, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ushijima, K. Treatment for recurrent ovarian cancer-at first relapse. J. Oncol. 2010, 2010, 497429. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Burleson, K.M.; Casey, R.C.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R., Jr.; Skubitz, A.P. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol. Oncol. 2004, 93, 170–181. [Google Scholar] [CrossRef]
- Al Habyan, S.; Kalos, C.; Szymborski, J.; McCaffrey, L. Multicellular detachment generates metastatic spheroids during intra-abdominal dissemination in epithelial ovarian cancer. Oncogene 2018, 37, 5127–5135. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef] [Green Version]
- Mulholland, T.; McAllister, M.; Patek, S.; Flint, D.; Underwood, M.; Sim, A.; Edwards, J.; Zagnoni, M. Drug screening of biopsy-derived spheroids using a self-generated microfluidic concentration gradient. Sci. Rep. 2018, 8, 14672. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; McArthur, C.; Jaffe, R.B. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br. J. Cancer 2010, 102, 1276–1283. [Google Scholar] [CrossRef] [Green Version]
- Miserocchi, G.; Mercatali, L.; Liverani, C.; De Vita, A.; Spadazzi, C.; Pieri, F.; Bongiovanni, A.; Recine, F.; Amadori, D.; Ibrahim, T. Management and potentialities of primary cancer cultures in preclinical and translational studies. J. Transl. Med. 2017, 15, 229. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, T.; Tardito, S. Cell Culture Medium Formulation and Its Implications in Cancer Metabolism. Trends Cancer 2019, 5, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Liberio, M.S.; Sadowski, M.C.; Soekmadji, C.; Davis, R.A.; Nelson, C.C. Differential effects of tissue culture coating substrates on prostate cancer cell adherence, morphology and behavior. PLoS ONE 2014, 9, e112122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Nalbantoglu, J. Ring cell migration assay identifies distinct effects of extracellular matrix proteins on cancer cell migration. BMC Res. Notes 2014, 7, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, A.; Howell, V.M.; Colvin, E.K. The Extracellular Matrix in Epithelial Ovarian Cancer—A Piece of a Puzzle. Front. Oncol. 2015, 5, 245. [Google Scholar] [CrossRef] [Green Version]
- Yoshiya, N. Establishment of a cell line from human ovarian cancer (undifferentiated carcinoma of FIGO classification) and analysis of its cell-biological characteristics and sensitivity to anticancer drugs. Nihon Sanka Fujinka Gakkai Zasshi 1986, 38, 1747–1753. [Google Scholar] [PubMed]
- Yoshiya, N.; Adachi, S.; Misawa, Y.; Yuzawa, H.; Honda, T.; Kanazawa, K.; Takeuchi, S.T.K.; Tanaka, K. Isolation of cisplatin-resistant subline from human ovarian cancer cell line and analysis of its cell-biological characteristics. Nihon Sanka Fujinka Gakkai Zasshi 1989, 41, 7–14. [Google Scholar]
- Schwartz, L.H.; Litiere, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Verschraegen, C.F.; Hu, W.; Du, Y.; Mendoza, J.; Early, J.; Deavers, M.; Freedman, R.S.; Bast, R.C., Jr.; Kudelka, A.P.; Kavanagh, J.J.; et al. Establishment and characterization of cancer cell cultures and xenografts derived from primary or metastatic Mullerian cancers. Clin. Cancer Res. 2003, 9, 845–852. [Google Scholar]
- Niedbala, M.J.; Crickard, K.; Bernacki, R.J. Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix. An in vitro model system for studying tumor cell adhesion and invasion. Exp. Cell Res. 1985, 160, 499–513. [Google Scholar] [CrossRef]
- Allen, H.J.; Porter, C.; Gamarra, M.; Piver, M.S.; Johnson, E.A. Isolation and morphologic characterization of human ovarian carcinoma cell clusters present in effusions. Exp. Cell Biol. 1987, 55, 194–208. [Google Scholar] [CrossRef]
- Woods, L.K.; Morgan, R.T.; Quinn, L.A.; Moore, G.E.; Semple, T.U.; Stedman, K.E. Comparison of four new cell lines from patients with adenocarcinoma of the ovary. Cancer Res. 1979, 39, 4449–4459. [Google Scholar] [PubMed]
- Ghani, F.I.; Dendo, K.; Watanabe, R.; Yamada, K.; Yoshimatsu, Y.; Yugawa, T.; Nakahara, T.; Tanaka, K.; Yoshida, H.; Yoshida, M.; et al. An Ex-Vivo Culture System of Ovarian Cancer Faithfully Recapitulating the Pathological Features of Primary Tumors. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kar, R.; Chawla, D.; Gupta, B.; Mehndiratta, M.; Wadhwa, N.; Agarwal, R. Establishment of Primary Cell Culture From Ascitic Fluid and Solid Tumor Obtained From Epithelial Ovarian Carcinoma Patients. Int. J. Gynecol. Cancer 2017, 27, 2000–2005. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Vidergar, R.; Belmonte, B.; Mangogna, A.; Amadio, L.; Geri, P.; Borelli, V.; Zanconati, F.; Tedesco, F.; Confalonieri, M.; et al. Complement Protein C1q Binds to Hyaluronic Acid in the Malignant Pleural Mesothelioma Microenvironment and Promotes Tumor Growth. Front. Immunol. 2017, 8, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzin, A.W.; Loret de Mola, J.R.; Bilker, W.B.; Wheeler, J.E.; Rubin, S.C.; Feinberg, R.F. Identification of oncofetal fibronectin in patients with advanced epithelial ovarian cancer: Detection in ascitic fluid and localization to primary sites and metastatic implants. Cancer 1998, 82, 152–158. [Google Scholar] [CrossRef]
- Varankar, S.S.; More, M.; Abraham, A.; Pansare, K.; Kumar, B.; Narayanan, N.J.; Jolly, M.K.; Mali, A.M.; Bapat, S.A. Functional balance between Tcf21-Slug defines cellular plasticity and migratory modalities in high grade serous ovarian cancer cell lines. Carcinogenesis 2019. [Google Scholar] [CrossRef]
- Kujawa, K.A.; Zembala-Nożyńska, E.; Cortez, A.J.; Kujawa, T.; Kupryjańczyk, J.; Lisowska, K.M. Fibronectin and Periostin as Prognostic Markers in Ovarian Cancer. Cells 2020, 9. [Google Scholar] [CrossRef] [Green Version]
- Masiello, T.; Dhall, A.; Hemachandra, L.P.M.; Tokranova, N.; Melendez, J.A.; Castracane, J. A Dynamic Culture Method to Produce Ovarian Cancer Spheroids under Physiologically-Relevant Shear Stress. Cells 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE 2014, 9, e84941. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Du, Y.; Lu, Y.; Luan, B.; Xu, C.; Yu, Y.; Zhao, H. CD44 Expression Predicts Prognosis of Ovarian Cancer Patients Through Promoting Epithelial-Mesenchymal Transition (EMT) by Regulating Snail, ZEB1, and Caveolin-1. Front. Oncol. 2019, 9, 802. [Google Scholar] [CrossRef] [Green Version]
- Iseki, Y.; Shibutani, M.; Maeda, K.; Nagahara, H.; Ikeya, T.; Hirakawa, K. Significance of E-cadherin and CD44 expression in patients with unresectable metastatic colorectal cancer. Oncol. Lett. 2017, 14, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Zheng, Y.; Liu, S.; Peng, Z. Fibronectin Regulates the Dynamic Formation of Ovarian Cancer Multicellular Aggregates and the Expression of Integrin Receptors. Asian Pac. J. Cancer Prev. 2018, 19, 2493–2498. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Roman, J. Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: Pro-oncogenic effects mediated by PI3-kinase and NF-kappa B. Oncogene 2006, 25, 4341–4349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, N.; Riley, C.; Rice, G.; Quinn, M. Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment. Clin. Exp. Metastasis 2005, 22, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Sakurai, H.; Ueno, Y.; Ohtani, O.; Saiki, I. Activation of MEK/ERK and PI3K/Akt pathways by fibronectin requires integrin alphav-mediated ADAM activity in hepatocellular carcinoma: A novel functional target for gefitinib. Cancer Sci. 2006, 97, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bragado, P.; Armesilla, A.; Silva, A.; Porras, A. Apoptosis by cisplatin requires p53 mediated p38alpha MAPK activation through ROS generation. Apoptosis 2007, 12, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, A.; Koster, R.; Boersma-van Eck, W.; Dam, W.A.; Mulder, N.H.; Gietema, J.A.; de Vries, E.G.; de Jong, S. Pro- and anti-apoptotic effects of p53 in cisplatin-treated human testicular cancer are cell context-dependent. Cell Cycle 2012, 11, 4552–4562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuertes, M.A.; Castilla, J.; Alonso, C.; Perez, J.M. Cisplatin biochemical mechanism of action: From cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr. Med. Chem. 2003, 10, 257–266. [Google Scholar] [CrossRef]
- Tanida, S.; Mizoshita, T.; Ozeki, K.; Tsukamoto, H.; Kamiya, T.; Kataoka, H.; Sakamuro, D.; Joh, T. Mechanisms of Cisplatin-Induced Apoptosis and of Cisplatin Sensitivity: Potential of BIN1 to Act as a Potent Predictor of Cisplatin Sensitivity in Gastric Cancer Treatment. Int. J. Surg. Oncol. 2012, 2012, 862879. [Google Scholar] [CrossRef]
- Guntur, V.P.; Waldrep, J.C.; Guo, J.J.; Selting, K.; Dhand, R. Increasing p53 protein sensitizes non-small cell lung cancer to paclitaxel and cisplatin in vitro. Anticancer Res. 2010, 30, 3557–3564. [Google Scholar]
- McDermott, M.; Eustace, A.J.; Busschots, S.; Breen, L.; Crown, J.; Clynes, M.; O’Donovan, N.; Stordal, B. In vitro Development of Chemotherapy and Targeted Therapy Drug-Resistant Cancer Cell Lines: A Practical Guide with Case Studies. Front. Oncol. 2014, 4, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldridge, B.B.; Burke, J.M.; Lauffenburger, D.A.; Sorger, P.K. Physicochemical modelling of cell signalling pathways. Nat. Cell Biol. 2006, 8, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Brigulova, K.; Cervinka, M.; Tosner, J.; Sedlakova, I. Chemoresistance testing of human ovarian cancer cells and its in vitro model. Toxicol. Vitr. 2010, 24, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Le Gallo, M.; de la Motte Rouge, T.; Poissonnier, A.; Lavoue, V.; Tas, P.; Leveque, J.; Godey, F.; Legembre, P. Tumor analysis: Freeze-thawing cycle of triple-negative breast cancer cells alters tumor CD24/CD44 profiles and the percentage of tumor-infiltrating immune cells. BMC Res. Notes 2018, 11, 401. [Google Scholar] [CrossRef]
- Menon, A.G.; Morreau, H.; Tollenaar, R.A.; Alphenaar, E.; Van Puijenbroek, M.; Putter, H.; Janssen-Van Rhijn, C.M.; Van De Velde, C.J.; Fleuren, G.J.; Kuppen, P.J. Down-regulation of HLA-A expression correlates with a better prognosis in colorectal cancer patients. Lab. Investig. 2002, 82, 1725–1733. [Google Scholar] [CrossRef] [Green Version]
- Trujillo, J.A.; Sweis, R.F.; Bao, R.; Luke, J.J. T Cell-Inflamed versus Non-T Cell-Inflamed Tumors: A Conceptual Framework for Cancer Immunotherapy Drug Development and Combination Therapy Selection. Cancer Immunol. Res. 2018, 6, 990–1000. [Google Scholar] [CrossRef] [Green Version]
- Schwertfeger, K.L.; Cowman, M.K.; Telmer, P.G.; Turley, E.A.; McCarthy, J.B. Hyaluronan, Inflammation, and Breast Cancer Progression. Front. Immunol. 2015, 6, 236. [Google Scholar] [CrossRef]
- Kurman, R.J.; International Agency for Research on Cancer; World Health Organization. WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2014; p. 307. [Google Scholar]
- Berek, J.S.; Kehoe, S.T.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 59–78. [Google Scholar] [CrossRef]
- Prat, J.; Oncology, F.C.o.G. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef]
- Vidergar, R.; Agostinis, C.; Zacchi, P.; Mangogna, A.; Bossi, F.; Zanconati, F.; Confalonieri, M.; Ricci, G.; Bulla, R. Evaluation of the Interplay Between the Complement Protein C1q and Hyaluronic Acid in Promoting Cell Adhesion. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]









| Patient Code | Age (Years) | Stage | Peritoneal Carcinosis | Ascites | Pleural Effusion | BRCA Germinal Mutation | CA125 Pre-Treatment (UI/L) |
|---|---|---|---|---|---|---|---|
| 1 | 64 | IIIC | + | + | - | no | 490 |
| 2 | 51 | IIIC | + | + | - | no | 168 |
| 3 | 68 | IIIC | + | + | - | yes | 649 |
| 4 | 58 | IIIC | + | + | - | no | 108 |
| 5 | 64 | IIIC | + | + | + | no | 1116 |
| 6 | 58 | IIIC | + | + | + | np | 118 |
| 7 | 76 | IIB | + | + | + | np | 7172 |
| 8 | 71 | IIIA | + | np | - | np | 20 |
| 9 | 55 | IIIA | + | - | - | yes | 290 |
| 10 | 73 | IIB | + | + | - | no | 1232 |
| 11 | 67 | IIB | - | - | - | yes | 16 |
| 12 | 69 | IIIC | + | + | - | np | 3175 |
| 13 | 60 | IIB | + | - | - | np | 70 |
| Patient Code | CA125 Pre-Treatment | CA125 Post-Treatment (3rd Cycle) | CA125 Post-Treatment (End) | Response Score | % Killing (CDDP 5 μg/mL) | |
|---|---|---|---|---|---|---|
| FN | HA | |||||
| 1 | 490 | 16 | 278 | −1 | 49.80 | 47.90 |
| 2 | 168 | 6 | <35 | 2 | 50.47 | 39.99 |
| 3 | 649 | 171 | <35 | 1 | 78.52 | 74.40 |
| 4 | 108 | 23 | <35 | 2 | 86.51 | 55.11 |
| 5 | 1116 | 32 | <35 | 2 | 35.79 | 48.64 |
| 6 | 118 | 45 | <35 | 0 | 37.17 | 51.51 |
| 7 | 7172 | 457 | 45 | 1 | 45.76 | 43.81 |
| 8 | 20 | np | <35 | 2 | 96.06 | 77.62 |
| 9 | 290 | 13 | 8 | 1 | 91.30 | 66.16 |
| 10 | 1232 | 625.9 | −1 | 57.76 | 1.55 | |
| 11 | 16 | np | 8.3 | 2 | 53.14 | 0.00 |
| 12 | 3175 | 43 | 58 | 1 | 71.83 | 52.40 |
| 13 | 70 | 6.5 | 5.6 | 2 | 36.61 | 32.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balduit, A.; Agostinis, C.; Mangogna, A.; Maggi, V.; Zito, G.; Romano, F.; Romano, A.; Ceccherini, R.; Grassi, G.; Bonin, S.; et al. The Extracellular Matrix Influences Ovarian Carcinoma Cells’ Sensitivity to Cisplatinum: A First Step towards Personalized Medicine. Cancers 2020, 12, 1175. https://doi.org/10.3390/cancers12051175
Balduit A, Agostinis C, Mangogna A, Maggi V, Zito G, Romano F, Romano A, Ceccherini R, Grassi G, Bonin S, et al. The Extracellular Matrix Influences Ovarian Carcinoma Cells’ Sensitivity to Cisplatinum: A First Step towards Personalized Medicine. Cancers. 2020; 12(5):1175. https://doi.org/10.3390/cancers12051175
Chicago/Turabian StyleBalduit, Andrea, Chiara Agostinis, Alessandro Mangogna, Veronica Maggi, Gabriella Zito, Federico Romano, Andrea Romano, Rita Ceccherini, Gabriele Grassi, Serena Bonin, and et al. 2020. "The Extracellular Matrix Influences Ovarian Carcinoma Cells’ Sensitivity to Cisplatinum: A First Step towards Personalized Medicine" Cancers 12, no. 5: 1175. https://doi.org/10.3390/cancers12051175
APA StyleBalduit, A., Agostinis, C., Mangogna, A., Maggi, V., Zito, G., Romano, F., Romano, A., Ceccherini, R., Grassi, G., Bonin, S., Bonazza, D., Zanconati, F., Ricci, G., & Bulla, R. (2020). The Extracellular Matrix Influences Ovarian Carcinoma Cells’ Sensitivity to Cisplatinum: A First Step towards Personalized Medicine. Cancers, 12(5), 1175. https://doi.org/10.3390/cancers12051175