Paclitaxel and Sorafenib: The Effective Combination of Suppressing the Self-Renewal of Cancer Stem Cells
Abstract
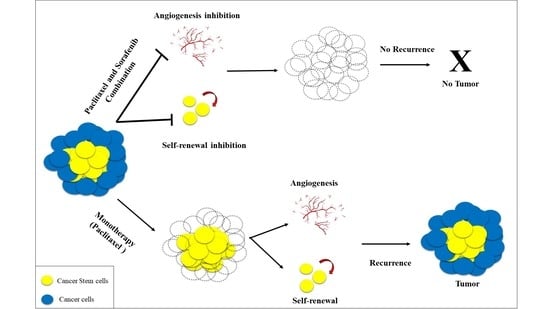
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Anticancer Drug and Inhibitor
2.3. Cell Proliferation and Viability Assay
2.4. Sphere Formation
2.5. Clonogenic Assay
2.6. In Vitro Tube Formation Assay
2.7. Apoptosis Analysis
2.8. Flow Cytometry
2.9. Chorioallantoic Membrane Assay
2.10. Statistical Analysis
3. Results
3.1. Synergistic Effect of PTX and Sor on Proliferation and Self-Renewal
3.2. Synergistic Effect of PTX and Sor on Colony Formation
3.3. Synergistic Effect of PTX and Sor on Angiogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CSCs | cancer stem cells |
| iPSCs | induced pluripotent stem cells |
| CM | conditioned medium |
| PTX | paclitaxel |
| Sor | sorafenib |
| miPS | mouse induced pluripotent stem cells |
| PBS | phosphate buffer solution |
| miPS-Huh7cmP | primary cultured cells isolated from liver tumor |
| miPS-Bt549cmP | primary cultured cells derived from tumor under skin |
| GFP | green fluorescent protein |
| LIF | leukemia inhibitory factor |
| Puro | puromycin |
| FBS | fetal bovine serum |
| DMSO | dimethyl sulfoxide |
| NEAA | non-essential amino acids |
| PI | propidium iodide |
| STR | profiling: short tandem repeat |
| hEGF | human epidermal growth factor |
| R3-IGF | R3-insulin-like growth factor-1 |
| hbFGF | human basic fibroblast growth factor |
| VEGF | vascular endothelial growth factor |
| GA | gentamicin/amphotericin B |
| CAM | chorioallantoic membrane assay |
References
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irène, B.; Andreas, T. The evolving concept of cancer and metastasis stem cells. J. Cell Biol. 2012, 198, 281–293. [Google Scholar]
- Corbin, E.M.; Sean, J.M. Tumor heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar]
- Afify, S.M.; Seno, M. Conversion of stem cells to cancer stem cells: Undercurrent of cancer initiation. Cancers 2019, 11, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-E.; Hsieh, C.-M.; Chen, L.-C.; Su, C.-Y.; Liu, D.-Z.; Jhan, H.-J.; Ho, H.-O.; Sheu, M.-T. Novel application of pluronic lecithin organogels (PLOs) for local delivery of synergistic combination of docetaxel and cisplatin to improve therapeutic efficacy against ovarian cancer. Drug Deliv. 2018, 25, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Zhang, Z.; Li, N.; Zhao, L.; Cai, M.; Sun, Z.; Li, Y.; Zhang, Y.; Khan, H.; Sun, H.; et al. HSA-based multi-target combination therapy: Regulating drugs’ release from HSA and overcoming single drug resistance in a breast cancer model. Drug Deliv. 2018, 25, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhu, X.; Han, M.; Hao, F.; Lu, W.; Zhou, T. Mechanistic Pharmacokinetic/ Pharmacodynamic Model of Sunitinib and Dopamine in MCF-7/Adr Xenografts: Linking Cellular Heterogeneity to Tumour Burden. AAPS J. 2020, 22, 45. [Google Scholar] [CrossRef]
- Gao, J.; Liu, J.; Xie, F.; Lu, Y.; Yin, C.; Shen, X. Co-Delivery of Docetaxel and Salinomycin to Target Both Breast Cancer Cells and Stem Cells by PLGA/TPGS Nanoparticles. Int. J. Nanomed. 2019, 14, 9199–9216. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Kasai, T.; Li, Y.; Sugii, Y.; Jin, G.; Okada, M.; Vaidyanath, A.; Mizutani, A.; Satoh, A.; Kudoh, T.; et al. A Model of Cancer Stem Cells Derived from Mouse Induced Pluripotent Stem Cells. PLoS ONE 2012, 7, e33544. [Google Scholar] [CrossRef] [Green Version]
- Calle, A.S.; Nair, N.; Oo, A.K.; Prieto-Vila, M.; Koga, M.; Khayrani, A.C.; Hussein, M.; Hurley, L.; Vaidyanath, A.; Seno, A.; et al. A new PDAC mouse model originated from iPSCs-converted pancreatic cancer stem cells (CSCcm). Am. J. Cancer Res. 2016, 6, 2799–2815. [Google Scholar] [PubMed]
- Nair, N.; Calle, A.S.; Zahra, M.H.; Prieto-Vila, M.; Oo, A.K.K.; Hurley, L.; Vaidyanath, A.; Seno, A.; Masuda, J.; Iwasaki, Y.; et al. A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Sci. Rep. 2017, 7, 6838. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Chen, L.; Yan, T.; Calle, A.S.; Nair, N.; Murakami, C.; Zahra, M.H.; Okada, N.; Iwasaki, Y.; Seno, A.; et al. Method to Convert Stem Cells into Cancer Stem Cells. Methods Protoc. 2019, 2, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afify, S.M.; Calle, A.; Hassan, G.; Kumon, K.; Nawara, H.; Zahra, M.; Mansour, H.; Khayrani, A.; Alam, M.; Juan, D.; et al. Novel model of liver cancer stem cells developed from induced pluripotent stem cells. Br. J. Cancer 2020, 122, 1378–1390. [Google Scholar] [CrossRef] [Green Version]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Zheng, B.; Wang, H.-Y.; Chen, L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharm. Sin. 2017, 38, 614–622. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tredan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [Green Version]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.G.; Lee, S.H.; Jun, N.; Kim, K.S.; Lee, Y.K. Cancer Stem Cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018, 2018, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yap, T.A.; Omlin, A.; de Bono, J.S. Development of therapeutic combinations targeting major cancer signaling pathways. J. Clin. Oncol. 2013, 31, 1592–1605. [Google Scholar] [CrossRef] [PubMed]
- Batson, S.; Mitchell, S.A.; Windisch, R.; Damonte, E.; Munk, V.C.; Reguart, N. Tyrosine kinase inhibitor combination therapy in first-line treatment of non–small cell lung cancer: Systematic review and network meta-analysis. Oncol. Targets Ther. 2017, 10, 2473–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, M.J.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K. EGFR TKI combination with immunotherapy in non–small cell lung cancer. Expert Opin. Drug Saf. 2017, 16, 465–469. [Google Scholar] [CrossRef] [PubMed]
- D’Cunha, R.; Bae, S.; Murry, D.J.; An, G. TKI combination therapy: Strategy to enhance dasatinib uptake by inhibiting Pgp- and BCRP-mediated efflux. Biopharm. Drug Dispos. 2016, 37, 397–408. [Google Scholar] [CrossRef]
- McGrogan, B.T.; Gilmartin, B.; Carney, D.N.; McCann, A. Taxanes, microtubules and chemoresistant breast cancer. Biochim. Biophys. Acta 2008, 1785, 96–132. [Google Scholar] [CrossRef]
- Demidenko, Z.N.; Kalurupalle, S.; Hanko, C.; Lim, C.-U.; Broude, E.; Blagosklonny, M.V. Mechanism of G1-like arrest by low concentrations of paclitaxel: Next cell cycle p53-dependent arrest with sub G1 DNA content mediated by prolonged mitosis. Oncogene 2008, 27, 4402–4410. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, G.; Liu, X.; Song, Y.; Xie, J.; Li, G.; Ren, J.; Wang, H.; Mou, J.; Dai, J.; et al. Sorafenib inhibited cell growth through the MEK/ERK signaling pathway in acute promyelocytic leukemia cells. Oncol. Lett. 2018, 15, 5620–5626. [Google Scholar]
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib blocks the RAF/MEK/erk pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar] [CrossRef] [Green Version]
- Merz, M.; Komljenovic, D.; Zwick, S.; Semmler, W.; Bäuerle, T. Sorafenib tosylate and paclitaxel induce anti-angiogenic, anti-tumour and anti-resorptive effects in experimental breast cancer bone metastases. Eur. J. Cancer 2011, 47, 277–286. [Google Scholar] [CrossRef]
- Choi, K.H.; Jeon, J.Y.; Lee, Y.-E.; Kim, S.W.; Kim, S.Y.; Yun, Y.J.; Park, K.C. Synergistic Activity of Paclitaxel, Sorafenib, and Radiation Therapy in advanced Renal Cell Carcinoma and Breast Cancer. Transl. Oncol. 2018, 12, 381–388. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawara, H.M.; Afify, S.M.; Hassan, G.; Zahra, M.H.; Atallah, M.N.; Mansour, H.; Abu Quora, H.A.; Alam, M.J.; Osman, A.; Kakuta, H.; et al. Paclitaxel and Sorafenib: The Effective Combination of Suppressing the Self-Renewal of Cancer Stem Cells. Cancers 2020, 12, 1360. https://doi.org/10.3390/cancers12061360
Nawara HM, Afify SM, Hassan G, Zahra MH, Atallah MN, Mansour H, Abu Quora HA, Alam MJ, Osman A, Kakuta H, et al. Paclitaxel and Sorafenib: The Effective Combination of Suppressing the Self-Renewal of Cancer Stem Cells. Cancers. 2020; 12(6):1360. https://doi.org/10.3390/cancers12061360
Chicago/Turabian StyleNawara, Hend M, Said M. Afify, Ghmkin Hassan, Maram H. Zahra, Marwa N Atallah, Hager Mansour, Hagar A. Abu Quora, Md Jahangir Alam, Amira Osman, Hiroki Kakuta, and et al. 2020. "Paclitaxel and Sorafenib: The Effective Combination of Suppressing the Self-Renewal of Cancer Stem Cells" Cancers 12, no. 6: 1360. https://doi.org/10.3390/cancers12061360
APA StyleNawara, H. M., Afify, S. M., Hassan, G., Zahra, M. H., Atallah, M. N., Mansour, H., Abu Quora, H. A., Alam, M. J., Osman, A., Kakuta, H., Hamada, H., Seno, A., & Seno, M. (2020). Paclitaxel and Sorafenib: The Effective Combination of Suppressing the Self-Renewal of Cancer Stem Cells. Cancers, 12(6), 1360. https://doi.org/10.3390/cancers12061360