Antibody-Drug Conjugates: A Promising Novel Therapy for the Treatment of Ovarian Cancer
Abstract
:1. Introduction
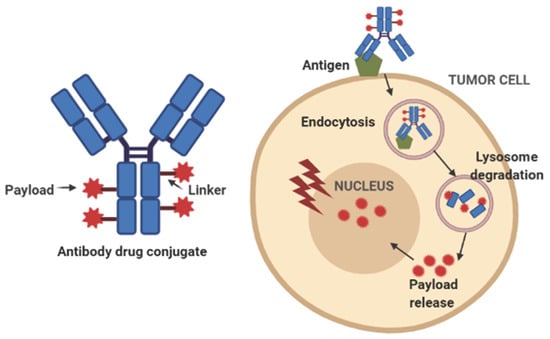
2. Structure of ADCs and Toxicity Profile
2.1. Antibody/Antigen
2.2. Payload
2.3. Linker
2.4. Toxicity Profile
3. ADCs in Ovarian Cancer
3.1. Anti-Folate Receptor Alpha-Based ADCs
3.2. Anti-NaPi2B-Based ADCs
3.3. Anti-MUC16-Based ADCs
3.4. Anti-Mesothelin-Based ADCs
3.5. Anti-Tissue Factor-Based ADCs
3.6. Other Antigen-Based ADCs
4. Mechanisms of Resistance to ADCs
5. Future Directions
6. Conclusions
Funding
Conflicts of Interest
References
- Lheureux, S.; Gourley, C.; Vergote, T.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Chellappan, D.K.; Leng, K.H.; Jia, L.J.; Aziz, N.A.B.A.; Hoong, W.C.; Qian, Y.C.; Ling, F.Y.; Wei, G.S.; Ying, T.; Chellian, J.; et al. The role of bevacizumab on tumour angiogenesis and in the management of gynaecological cancers: A review. Biomed. Pharm. 2018, 102, 1127–1144. [Google Scholar] [CrossRef] [PubMed]
- Ruscito, I.; Belleti, F.; Ray-Coquard, I.; Mirza, M.R.; du Bois, A.; Gasparri, M.L.; Costanzi, F.; De Marco, M.P.; Nuti, M.; Caserta, D.; et al. Incorporating parp-inhibitors in primary and recurrent ovarian cancer: A meta-analysis of 12 phase II/III randomized controlled trials. Cancer Treat. Rev. 2020, 87, 102040. [Google Scholar] [CrossRef]
- Carter, P.; Lazar, G.A. Next generation antibody drugs: Pursuit of the ‘High-Hanging Fruit’. Nat. Rev. Drug Discov. 2018, 17, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumomtet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–317. [Google Scholar] [CrossRef]
- Tolcher, A.W. The evolution of antibody-drug conjugates: A positive inflexion point. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 1–8. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sacituzumab govitecan: First approval. Drugs 2020, 80, 1019–1025. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryvkov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Teicher, B.A.; Chari, R.V. Antibody conjugate therapeutics: Challenges and potential. Clin. Cancer Res. 2011, 17, 6389–6397. [Google Scholar] [CrossRef] [Green Version]
- Doronina, S.O.; Toki, B.E.; Torgov, M.Y.; Mendelsohn, B.A.; Cerveny, C.G.; Chace, D.F.; Deblanc, R.L.; Gearing, R.P.; Bovee, T.D.; Siegall, C.B.; et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 2003, 21, 778–784. [Google Scholar] [CrossRef]
- Erickson, H.K.; Park, P.U.; Widdison, W.C.; Kovtun, Y.V.; Garrett, L.M.; Hoffman, K.; Lutz, R.J.; Goldmacher, V.S.; Blättler, W.A. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006, 66, 4426–4433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.K.; Liu, J.F. Antibody-drug conjugates in gynecologic malignancies. Gynecol. Oncol. 2019, 153, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Borghaei, H.; O’Malley, D.M.; Jeong, W.; Seward, S.M.; Bauer, T.M.; Perez, R.P.; Matulonis, U.A.; Running, K.L.; Zhang, X.; et al. Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor α-targeting antibody-drug conjugate, in patients with solid tumors. Cancer 2017, 123, 3080–3087. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Martin, L.P.; O’Malley, D.M.; Matulonis, U.A.; Konner, J.A.; Perez, R.P.; Bauer, T.M.; Ruiz-Soto, R.; Birrer, M.J. Safety and activity of mirvetuximab soravtansine (IMGN853), a Folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: A phase I expansion study. J. Clin. Oncol. 2017, 35, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Vergote, I.; Oaknin, A.; Colombo, N.; Banerjee, S.; Oza, A.; Pautier, P.; Malek, K.; Birrer, M.J. FORWARD I: A phase III study of mirvetuximab soravtansine versus chemotherapy in platinum-resistant ovarian cancer. Future Oncol. 2018, 14, 1669–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, K. FORWARD I (GOG 3011): A phase III study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), versus chemotherapy in patients (pts) with platinum-resistant ovarian cancer (PROC). ESMO Abstract #992O). Ann. Oncol. 2019, 30, v403. [Google Scholar]
- O’Malley, D.M.; Matulonis, U.A.; Birrer, M.J.; Castro, C.M.; Gilbert, L.; Vergote, I.; Martin, L.P.; Mantia-Smaldone, G.M.; González-Martín, A.; Bratos, R.; et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 2020, 157, 379–385. [Google Scholar] [CrossRef]
- Moore, K.N.; O’Malley, D.M.; Vergote, I.; Martin, L.P.; Gonzalez-Martin, A.; Malek, K.; Birrer, M.J. Safety and activity findings from a phase 1b escalation study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with carboplatin in patients with platinum-sensitive ovarian cancer. Gynecol. Oncol. 2018, 151, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Oza, A.M.; Birrer, M.J.; Hamilton, E.P.; Hasan, J.; Leary, A.; Moore, K.; Mackowiak-Matejczyk, B.; Pikiel, J.; Ray-Coquard, I.; et al. Anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin (DNIB0600A) Compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer in a randomized, open-label, phase II study. Ann. Oncol. 2018, 29, 917–923. [Google Scholar] [CrossRef]
- Liu, J.F.; Moore, K.N.; Birrer, M.J.; Berlins, S.; Matulonis, U.A.; Infante, J.R.; Wolpin, B.; Poon, K.A.; Firestein, R.; Xu, J.; et al. Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody-drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann. Oncol. 2016, 27, 2124–2130. [Google Scholar] [CrossRef]
- Bulat, I.; Moore, K.N.; Hacetrean, A.; Chung, J.W.; Rajagopalan, P.; Xia, C.; Laurent, D.; Childs, B.H.; Santin, A. Phase Ib study of anti-mesothelin antibody drug conjugate anetumab ravtansine in combination with pegylated liposomal doxorubicin in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer. J. Clin. Oncol. 2018, 36 (Suppl. 5571), 5571. [Google Scholar] [CrossRef]
- De Bono, J.S.; Concin, N.; Hong, D.S.; Thistlethwaite, F.C.; Machiels, J.-P.; Arkenau, H.-T.; Plummer, R.; Jones, R.H.; Nielsen, D.; Windfeld, K.; et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): A first-in-human, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 383–393. [Google Scholar] [CrossRef]
- Damelin, M.; Bankovich, A.; Bernstein, J.; Lucas, J.; Chen, L.; Williams, S.; Park, A.; Aguilar, J.; Ernstoff, E.; Charati, M.; et al. A PTK7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci. Transl. Med. 2017, 9, eaag2611. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.J.; Vitale, L.; O´Neill, T.Y.; Dolnick, R.; Wallace, P.K.; Minderman, H.; E Gergel, L.; Forsberg, E.M.; Boyer, J.M.; Storey, J.R.; et al. Development of a novel antibody-drug conjugate for the potential treatment of ovarian, lung, and renal cell carcinoma expressing TIM-1. Mol. Cancer Rev. 2016, 15, 2946–2954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, D.M.; Sharkey, R.M. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert. Opin. Biol. 2020, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.S.; Wesolowski, R.; Baffa, R.; Liao, K.H.; Hua, S.Y.; Gibson, B.L.; Pirie-Shepherd, S.; Tolcher, A.W. A phase I, dose-escalation study of PF-06650808, an anti-Notch3 antibody-drug conjugate, in patients with breast cancer and other advanced solid tumors. Investig. New Drugs 2020, 38, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Boni, V.; Burris, H.A., III; Liu, J.F.; Spira, A.I.; Arkenau, H.T.; Fidle, M.J.; Rosen, L.S.; Sweis, R.F.; Uboha, N.V.; Sanborn, R.E.; et al. CX-2009, a CD166-directed probody drug conjugate (PDC): Results from the first-in-human study in patients (Pts) with advanced cancer including breast cancer (BC). J. Clin. Oncol. 2020, 38, 526. [Google Scholar] [CrossRef]
- Ducry, L.; Stump, B. Antibody−drug conjugates: Linking cytotoxic payloads to monoclonal antibodies. Bioconjug. Chem. 2010, 21, 5–13. [Google Scholar] [CrossRef]
- Hamblett, J.; Senter, P.D.; Chace, D.F.; Sun, M.M.C.; Lenox, J.; Cerveny, C.G.; Kissler, K.M.; Bernhardt, S.X.; Kopcha, A.K.; Zabinski, R.F.; et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 2004, 10, 7063–7070. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.; Smith, S.W.; Ghone, S.; Tomczuk, B. Current ADC linker chemistry. Pharm. Res. 2015, 32, 3526–3540. [Google Scholar] [CrossRef] [Green Version]
- Bargh, J.D.; Isidro-Llobet, A.; Parker, J.S.; Spring, D.R. Cleavable linkers in antibody-drug conjugates. Chem. Soc. Rev. 2019, 48, 4361–4374. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, Y.V.; Audette, C.A.; Ye, Y.; Xie, H.; Ruberti, M.F.; Phinney, S.J.; Leece, B.A.; Chittenden, T.; Blättler, W.A.; Goldmacher, V.S. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006, 66, 3214–3221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2018, 9, 33–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaghy, H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibodydrug conjugates. MAbs 2016, 8, 659–671. [Google Scholar] [CrossRef]
- Rudmann, D.G. On-target and off-target-based toxicology effects. Toxicol. Pathol. 2013, 41, 310–314. [Google Scholar] [CrossRef]
- Masters, J.C.; Nickens, D.J.; Xuan, D.; Shazer, R.L.; Amantea, M. Clinical toxicity of antibody drug conjugates: A meta-analysis of payloads. Investig. New Drugs 2018, 36, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gulesserian, S.; Malinao, M.C.; Ganesan, S.K.; Song, J.; Chang, M.S.; Zeng, Z.; Mattie, M.D.; Doñate, F.; Williams, M.M.; et al. A Potential mechanism for ADC-induced neutropenia: Role of neutrophils in their own demise. Mol. Cancer Ther. 2017, 16, 1866–1876. [Google Scholar] [CrossRef] [Green Version]
- Stagg, N.J.; Shen, B.Q.; Brunstein, F.; Li, C.; Kamath, A.V.; Zhong, F.; Schutten, M.; Fine, B. Peripheral neuropathy with microtubule inhibitor containing antibody drug conjugates: Challenges and perspectives in translatability from nonclinical toxicology studies to the clinic. Regul. Toxicol. Pharmacol. 2016, 82, 1–13. [Google Scholar] [CrossRef]
- Eaton, J.S.; Miller, P.E.; Mannis, M.J.; Murphy, C.J. Ocular adverse events associated with antibody drug conjugates in human clinical trials. J. Ocul. Pharm. Ther. 2015, 31, 589–604. [Google Scholar] [CrossRef]
- Matunolis, U.A.; Birrer, M.J.; O’Malley, D.M.; Moore, K.N.; Konner, J.; Glibert, L.; Martin, L.P.; Bauer, T.M.; Oza, A.M.; Malek, K.; et al. Evaluation of prophylactic corticosteroid eye drop use in the management of corneal abnormalities induced by the antibody-drug conjugate mirvetuximab soravtansine. Clin. Cancer Res. 2019, 25, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- Govindarajan, B.; Gipson, I.K. Membrane-tethered mucins have multiple functions on the ocular surface. Exp. Eye Res. 2010, 90, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uyar, D.; Schilder, R.; Naumann, R.W.; Braiteh, F.; Hamilton, E.; Diab, S.; Moroney, J.; Penson, R.T.; Smith, J.; Abrahams, C.; et al. Antitumor activity of STRO-002, a novel anti-folate receptor-α (FRα) antibody drug conjugate (ADC), in patient-derived xenograft (PDX) models and preliminary Phase I dose escalation safety outcomes in patients with ovarian carcinoma (OC).C095. Poster session C: Tubulin-interacting agents. In Proceedings of the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics Conference, Boston, MA, USA, 26–30 October 2019. [Google Scholar]
- Shimizu, T.; Fujiwara, Y.; Yonemori, K.; Koyama, T.; Shimomura, A.; Tamura, K.; Iwasa, S.; Sato, J.; Kitano, S.; Ikezawa, H.; et al. First-in-human (FIH) phase 1 (Ph1) study of MORAb-202 in patients (pts) with advanced folate receptor alpha (FRA) positive solid tumors. J. Clin. Oncol. 2019, 37, 5544. [Google Scholar] [CrossRef]
- Weekes, C.D.; Lamberts, L.E.; Borad, M.J.; Voortman, J.; McWilliams, R.R.; Diamond, J.R.; De Vries, E.G.; Verheul, H.M.; Lieu, C.; Kim, G.P.; et al. Phase I study of DMOT4039A, an antibody-drug conjugate targeting mesothelin, in patients with unresectable pancreatic or platinum-resistant ovarian cancer. Mol. Cancer 2016, 15, 439–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, K.; Hamilton, E.P.; Burris, H.A.; Barroilhet, L.M.; Gutierrez, M.; Wang, J.S.; Patel, M.R.; Birrer, M.J.; Flanagan, W.M.; Wang, Y.; et al. Targeting MUC16 with the THIOMABTM- drug conjugate DMUC4064A in patients with platinum-resistant ovarian cancer: A phase I expansion study. In Proceedings of the 109th Annual Meeting of the American Association for Cancer Research, Chicago, IL, USA, 14–18 April 2018. [Google Scholar]
- Tolcher, A.W.; Ulahannan, S.V.; Papadopoulos, K.P.; Edenfield, W.J.; Matulonis, U.A.; Burns, T.F.; Mosher, R.; Fielman, B.; Hailman, E.; Burris, H.A.; et al. Phase 1 dose escalation study of XMT-1536, a novel NaPi2b-targeting antibody-drug conjugate (ADC), in patients with solid tumors likely to express NaPi2b. J. Clin. Oncol. 2019, 37, 3010. [Google Scholar] [CrossRef]
- Elnakat, H.; Ratnam, M. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv. Drug. Deliv. Rev. 2004, 56, 1067–1084. [Google Scholar] [CrossRef]
- Kalli, K.R.; Oberg, A.L.; Keeney, G.L.; Christianson, T.J.; Low, P.S.; Knutson, K.L.; Hartmann, L.C. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol. Oncol. 2008, 108, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Matulonis, U.; Moore, K.N.; Martin, L.P.; Vergote, I.B.; Castro, C.; Gilbert, L.; Malek, K.; Birrer, M.; O’Malley, D. Mirvetuximab soravtansine, a folate receptor alpha (FR)-targeting antibody-drug conjugate (ADC), with pembrolizumab in platinum-resistant ovarian cancer (PROC): Initial results of an expansion cohort from FORWARD II, a phase IB study. Ann. Oncol. 2018, 29, viii339. [Google Scholar] [CrossRef]
- Feild, J.L.; Brun, Z.K.; Brooks, D.P.; Edwards, R.M. Cloning and functional char-acterization of a sodium-dependent phosphate transporter expressed in human lung and small intestine. Biochem. Biophys. Res. Commun. 1999, 258, 578–582. [Google Scholar] [CrossRef]
- Levan, K.; Mehryar, M.; Mateoiu, C.; Albertsson, P.; Back, T.; Sundfeldt, K. Immunohistochemical evaluation of epithelial ovarian carcinomas identifies three different expression patterns of the MX35 antigen, NaPi2b. BMC Cancer 2017, 17, 303. [Google Scholar] [CrossRef]
- Boivin, M.; Lane, D.; Piché, A.; Rancourt, C. CA125 (MUC16) tumor antigen selectively modulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecol. Oncol. 2009, 115, 407–413. [Google Scholar] [CrossRef]
- Hassan, R.; Kreitman, R.J.; Pastan, I.; Willingham, M.C. Localization of mesothelin in epithelial ovarian cancer. Appl. Immunohistochem. Mol. Morphol. 2005, 13, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N.; Tilley, R.E.; Key, N.S. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1687–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocco, E.; Varughese, J.; Buza, N.; Bellone, S.; Lin, K.Y.; Bellone, M.; Todeschini, P.; Silasi, D.-A.; Azodi, M.; Schwartz, P.E.; et al. Tissue factor expression in ovarian cancer: Impli-cations for immunotherapy with hI-con1, a factor VII-IgGF(c) chimeric protein targeting tissue factor. Clin. Exp. Metastasis 2011, 28, 689–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Autio, K.A.; Boni, V.; Humphrey, R.W.; Naing, A. Probody therapeutics: An emerging class of therapies designed to enhance on-target effects with reduced off-tumor toxicity for use in immuno-oncology. Clin. Cancer Res. 2020, 26, 984–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Alonso, S.; Ocana, A.; Pandiella, A. Resistance to antibody-drug conjugates. Cancer Res. 2018, 78, 2159–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Hou, J.; Newman, E.; Kim, Y.; Donohue, C.; Liu, X.; Thomas, S.H.; Forman, S.J.; Kane, S.E. CD30 downregulation, MMAE resistance, and MDR1 upregulation are all associated with resistance to brentuximab vedotin. Mol. Cancer Ther. 2015, 14, 1376–1384. [Google Scholar] [CrossRef] [Green Version]
- Al-Rohil, R.N.; Torres-Cabala, C.A.; Patel, A.; Tetzlaff, M.T.; Ivan, D.; Nagarajan, P.; Curry, J.L.; Miranda, R.N.; Duvic, M.; Prieto, V.G.; et al. Loss of CD30 expression after treatment with brentuximab vedotin in a patient with anaplastic large cell lymphoma: A novel finding. J. Cutan. Pathol. 2016, 43, 1161–1166. [Google Scholar] [CrossRef]
- Scaltriti, M.; Rojo, F.; Ocana, A.; Anido, J.; Guzman, M.; Cortes, J.; Di Cosimo, S.; Matías-Guiu, X.; Cajal, S.R.Y.; Arribas, J.; et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J. Natl. Cancer Inst. 2007, 99, 628–638. [Google Scholar] [CrossRef] [Green Version]
- Sung, M.; Tan, X.; Lu, B.; Golas, J.; Hosselet, C.; Wang, F.; Tylaska, L.; King, L.; Zhou, D.; Dushin, R.; et al. Caveolae-Mediated Endocytosis as a Novel Mechanism of Resistance to Trastuzumab Emtansine (T-DM1). Mol. Cancer Ther. 2017, 17, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Ríos-Luci, C.; García-Alonso, S.; Díaz-Rodríguez, E.; Arribas, J. Resistance to the antibody-drug conjugate T-DM1 is based in a reduction in lysosomal proteolytic activity. Cancer Res. 2017, 77, 4639–4651. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, M.; Tchistiakova, L.; Scott, N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs 2013, 5, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavallaris, M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 2010, 10, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Chen, C.; Tammali, R.; Breen, S.; Zhang, J.; Fazenbaker, C.; Kennedy, M.; Conway, J.; Higgs, B.W.; Holoweckyj, N.; et al. Improved therapeutic window in BRCA-mutant tumors with antibody-linked pyrrolobenzodiazepine dimers with and without PARP inhibition. Mol. Cancer Ther. 2019, 18, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, P.; Martin, K.; Theurich, S.; Schreiner, J.; Savic, S.; Terszowski, G.; Lardinois, D.; Heinzelmann-Schwarz, V.; Schlaak, M.; Kvasnicka, H.M.; et al. Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol. Res. 2014, 2, 741–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios-Doria, J.; Harper, J.; Rothstein, R.; Wetzel, L.; Chesebrough, J.; Marrero, A.M.; Chen, C.; Strout, P.; Mulgrew, K.; A McGlinchey, K.; et al. Antibody-drug conjugates bearing pyrrolobenzodiazepine or tubulysin payloads are immunomodulatory and synergize with multiple immunotherapies. Cancer Res. 2017, 77, 2686–2698. [Google Scholar] [CrossRef] [Green Version]

| ADC | Antigen | Payload | Class | Mechanism of Action | Linker and DAR | Development Stage |
|---|---|---|---|---|---|---|
| Mirvetuximab soravtansine [13,14,15,16,17,18] | Folate receptor alpha | DM4 | Maytansinoid | Microtubule-disrupting agent | Cleavable 3–4 | Phase 3 |
| Lifastuzumab vedotin [19] | NaPi2B | monomethyl auristatin E (MMAE) | Auristatin analogs | Microtubule-disrupting agent | Cleavable 3–4 | Phase 2 (discontinued) |
| Sofituzumab vedotin [20] | MUC16 | monomethyl auristatin E (MMAE) | Auristatin analogs | Microtubule-disrupting agent | Cleavable 3.5 | Phase 1 (discontinued) |
| Anetumab ravtansine [21] | Mesothelin | DM4 | Maytansinoid | Microtubule-disrupting agent | Cleavable 3.2 | Phase 2 |
| Tisotumab vedotin [22] | Tissue factor | monomethyl auristatin E (MMAE) | Auristatin analogs | Microtubule-disrupting agent | Cleavable NR | Phase 2 |
| Cofituzumab pelidotin [23] | PTK7 | Aur0101 | Auristatin analogs | Microtubule-disrupting agent | Cleavable NR | Preclinical |
| CDX-014 [24] | TIM1 | monomethyl auristatin E (MMAE) | Auristatin analogs | Microtubule-disrupting agent | Cleavable 4.5 | Preclinical |
| Sacituzumab govitecan [25] | TROP-2 | SN-38 | Camptothecin | Topoisomerase inhibitor analog | Cleavable 6.78 | Preclinical |
| PF-06650808 [26] | NOTCH-3 | monomethyl auristatin E (MMAE) | Auristatin analogs | Microtubule-disrupting agent | Cleavable NR | Phase 1 |
| Praluzatamab ravtansine, CX-2009 [27] | CD166 | DM4 | Maytansinoid | Microtubule-disrupting agent | Cleavable 3.5 | Phase 1 |
| Antigen | Function | Expression in Normal Cells | Expression in Ovarian Cancer Cells | ADC | Payload |
|---|---|---|---|---|---|
| FRα | Intracellular transport of folate | Marginally expressed in normal cells (polarized epithelium) | 67–100% | Mirvetuximab soravtansine (IMGN853) | DM4 |
| Mesothelin | Cell adhesion | Expressed in pleura, peritoneum and pericardium | 55–100% | Anetumab ravtansine | DM4 |
| Tissue factor | Extrinsic pathway of the coagulation cascade | Subendothelial vessel wall cells | 23–100% | Tisotumab vedotin | MMAE |
| MUC16 | Protection of epithelial surfaces | Epithelial cells (eye, mesothelium, trachea) | 70–90% | Sofituzumab vedotin | MMAE |
| TROP2 | Intracellular calcium signal transducer | Trophoblast cells, alveolar epithelial cells, smooth muscle cells | 82–92% | Sacituzumab govitecan | SN-38 |
| NaPi2B | Sodium-dependent surface transporter | Epithelial cells (pneumocytes, small bowel, mammary gland) | 80–93% | Lifastuzumab vedotin (LIFA) | MMAE |
| ADCs | Target Antigen | Phase of Development | Efficacy of Monotherapy | Efficacy in Combination | Main Toxicity (>20%) |
|---|---|---|---|---|---|
| Mirvetuximab soravtansine [13,14,15,16,17,18] | FRα | Phase III | ORR 24–46% mPFS 4.8–6.7 months | Bev (platinum resistant): ORR 39% Carbo AUC4–5 (platinum sensitive): ORR 71%, mPFS 15 months | Ocular toxicity (blurred vision, keratopathy), neurotoxicity, fatigue, AST increased, nausea |
| STRO-002 [42] | FRα | Phase 1 | ongoing | – | Fatigue, vomiting, decreased appetite, constipation, AST increased, neuropathy |
| MORAb-202 [43] | FRα | Phase 1 | DCR 75% (1/9 CR; 2/9 PR) | – | ALT and GGT increased, leukopenia, neutropenia |
| Anetumab ravtansine [21] | Mesothelin | Phase 1b–2 | ORR 9% DCR 59% | PLD: DCR 83% (52% PR, 33% SD) | Keratitis and neuropathy (both DLT). GI disorders |
| DMOT4039A [44] | Mesothelin | Phase 1 | ORR 30% mPFS4.9 months | – | Diarrhea, nausea, fatigue, alopecia |
| Tisotumab vedotin [22] | Tissue factor | Phase 1–2 | ORR 13.9% | – | Ocular toxicity (conjunctivitis, dry eye), epistaxis, fatigue, neuropathy, nausea, diarrhea, decreased appetite |
| Sofituzumab vedotin [20] | MUC16 | Phase 1 | ORR 17% | – | Fatigue, neuropathy, nausea, decreased appetite, diarrhea, alopecia, pyrexia, anemia, neutropenia, hypomagnesemia |
| DMU4C064A [45] | MUC16 | Phase 1 | ORR 45% (1CR/8 PR) mPFS 5.8 months | – | Ocular toxicity (visual disturbance, keratitis, dry eye), neuropathy, diarrhea, nausea, fatigue |
| Lifastuzumab vedotin (LIFA) [19] | NaPi2B | Phase 2 | ORR 34% vs.15% (p = 0.03) mPFS 5.3 vs. 3.1 months (HR 0.71) | – | Neuropathy, diarrhea, nausea, constipation, neutropenia, anemia, fatigue |
| XMT1536 [46] | NaPi2B | Phase 1 | 2 CR, 11 prolonged SD | – | Nausea, fatigue, headache |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzano, A.; Ocaña, A. Antibody-Drug Conjugates: A Promising Novel Therapy for the Treatment of Ovarian Cancer. Cancers 2020, 12, 2223. https://doi.org/10.3390/cancers12082223
Manzano A, Ocaña A. Antibody-Drug Conjugates: A Promising Novel Therapy for the Treatment of Ovarian Cancer. Cancers. 2020; 12(8):2223. https://doi.org/10.3390/cancers12082223
Chicago/Turabian StyleManzano, Aranzazu, and Alberto Ocaña. 2020. "Antibody-Drug Conjugates: A Promising Novel Therapy for the Treatment of Ovarian Cancer" Cancers 12, no. 8: 2223. https://doi.org/10.3390/cancers12082223
APA StyleManzano, A., & Ocaña, A. (2020). Antibody-Drug Conjugates: A Promising Novel Therapy for the Treatment of Ovarian Cancer. Cancers, 12(8), 2223. https://doi.org/10.3390/cancers12082223