Impact of Number of Segmented Tissues on SAR Prediction Accuracy in Deep Pelvic Hyperthermia Treatment Planning
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Models Selection
2.2. Tissue Segmentation Lists
2.3. SAR-Based Hyperthermia Treatment Planning
2.4. Dosimetry Evaluation
3. Results
3.1. Representative Value of the Virtual Family Models
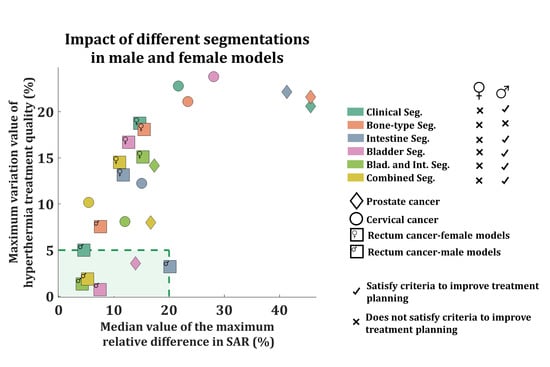
3.2. Impact of Tissue Segmentation on Treatment Quality (|dTHQ|)
3.3. Impact of Tissue Segmentation on SAR Prediction (|dRD|)
4. Discussion
4.1. Study Results
4.2. Clinical Translation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cihoric, N.; Tsikkinis, A.; Van Rhoon, G.; Crezee, H.; Aebersold, D.M.; Bodis-Wollner, I.; Beck, M.; Nadobny, J.; Budach, V.; Wust, P.; et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int. J. Hyperth. 2015, 31, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Stutz, E.; Gomez, S.; Bodis, S. Efficacy and Safety Evaluation of the Various Therapeutic Options in Locally Advanced Cervix Cancer: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials. Int. J. Radiat. Oncol. 2019, 103, 411–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Zee, J. Heating the patient: A promising approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef]
- Kok, H.P.; Beck, M.; Löke, D.R.; Helderman, R.F.C.P.A.; Van Tienhoven, G.; Ghadjar, P.; Wust, P.; Crezee, H. Locoregional peritoneal hyperthermia to enhance the effectiveness of chemotherapy in patients with peritoneal carcinomatosis: A simulation study comparing different locoregional heating systems. Int. J. Hyperth. 2020, 37, 76–88. [Google Scholar] [CrossRef]
- International collaborative hypert; Vernon, C.; Hand, J.; Field, S.; Machin, D.; Whaley, J.; Zee, J.; Van Putten, W.; Vanrhoon, G.; Vandijk, J. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. Int. J. Radiat. Oncol. 1996, 35, 731–744. [Google Scholar] [CrossRef] [Green Version]
- Overgaard, J.; Bentzen, S.M.; González, D.G.; Hulshof, M.C.C.M.; Arcangeli, G.; Dahl, O.; Mella, O. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet Oncol. 1995, 345, 540–543. [Google Scholar] [CrossRef]
- Issels, R.; Lindner, L.H.; Verweij, J.; Wust, P.; Reichardt, P.; Schem, B.-C.; Abdel-Rahman, S.; Daugaard, S.; Salat, C.; Wendtner, C.-M.; et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010, 11, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Zee, J.; Van Der González, D.G.; Rhoon, G.C.; Van Dijk, J.D.P.; Van Putten, W.L.J. Van Comparison of Radiotherapy alone with Radiotherapy plus Hyperthermia in Locally Advanced Pelvic Tumors. Lancet 2000, 355, 1119–1125. [Google Scholar]
- Peeken, J.C.; Vaupel, P.; Combs, S.E. Integrating Hyperthermia into Modern Radiation Oncology: What Evidence Is Necessary? Front. Oncol. 2017, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Franckena, M.; Fatehi, D.; de Bruijne, M.; Canters, R.A.M.; van Norden, Y.; Mens, J.W.; van Rhoon, G.C.; van der Zee, J. Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur. J. Cancer 2009, 45, 1969–1978. [Google Scholar] [CrossRef]
- Fatehi, D.; Van Der Zee, J.; Notenboom, A.; Van Rhoon, G.C. Comparison of Intratumor and Intraluminal Temperatures During Locoregional Deep Hyperthermia of Pelvic Tumors. Strahlenther. Onkol. 2007, 183, 479–486. [Google Scholar] [CrossRef]
- Kroesen, M.; Mulder, H.T.; Van Holthe, J.M.; Aangeenbrug, A.A.; Mens, J.W.M.; Van Doorn, H.C.; Paulides, M.M.; Hoop, E.O.-D.; Vernhout, R.M.; Lutgens, L.C.; et al. Confirmation of thermal dose as a predictor of local control in cervical carcinoma patients treated with state-of-the-art radiation therapy and hyperthermia. Radiother. Oncol. 2019, 140, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.P.; Wust, P.; Stauffer, P.R.; Bardati, F.; Van Rhoon, G.C.; Crezee, H. Current state of the art of regional hyperthermia treatment planning: A review. Radiat. Oncol. 2015, 10, 196. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasa, G.; Gellermann, J.; Rau, B.; Nadobny, J.; Schlag, P.; Deuflhard, P.; Felix, R.; Wust, P. Clinical use of the hyperthermia treatment planning system HyperPlan to predict effectiveness and toxicity. Int. J. Radiat. Oncol. 2003, 55, 407–419. [Google Scholar] [CrossRef]
- Bruggmoser, G. Some aspects of quality management in deep regional hyperthermia. Int. J. Hyperth. 2012, 28, 562–569. [Google Scholar] [CrossRef]
- Kok, H.P.; Schooneveldt, G.; Bakker, A.; De Kroon-Oldenhof, R.; Straten, L.K.-V.; De Jong, C.E.; Steggerda-Carvalho, E.; Geijsen, E.D.; A Stalpers, L.J.; Crezee, H. Predictive value of simulated SAR and temperature for changes in measured temperature after phase-amplitude steering during locoregional hyperthermia treatments. Int. J. Hyperth. 2018, 35, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Franckena, M.; Canters, R.; Termorshuizen, F.; Van Der Zee, J.; Van Rhoon, G. Clinical implementation of hyperthermia treatment planning guided steering: A cross over trial to assess its current contribution to treatment quality. Int. J. Hyperth. 2010, 26, 145–157. [Google Scholar] [CrossRef]
- Lagendijk, J.J.W. Hyperthermia treatment planning. Phys. Med. Biol. 2000, 45, R61. [Google Scholar] [CrossRef]
- Kok, H.P.; Ciampa, S.; De Kroon-Oldenhof, R.; Steggerda-Carvalho, E.J.; Van Stam, G.; Vörding, P.J.Z.V.S.; A Stalpers, L.J.; Geijsen, E.D.; Bardati, F.; Bel, A.; et al. Toward Online Adaptive Hyperthermia Treatment Planning: Correlation Between Measured and Simulated Specific Absorption Rate Changes Caused by Phase Steering in Patients. Int. J. Radiat. Oncol. 2014, 90, 438–445. [Google Scholar] [CrossRef]
- De Greef, M.; Kok, H.P.; Correia, D.; Bel, A.; Crezee, H. Optimization in hyperthermia treatment planning: The impact of tissue perfusion uncertainty. Med. Phys. 2010, 37, 4540–4550. [Google Scholar] [CrossRef] [Green Version]
- Bruggmoser, G.; Bauchowitz, S.; Canters, R.; Crezee, H.; Ehmann, M.; Gellermann, J.; Lamprecht, U.; Lomax, N.; Messmer, M.; Ott, O.; et al. Guideline for the clinical application, documentation and analysis of clinical studies for regional deep hyperthermia. Strahlenther. Onkol. 2012, 188, 198–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagendijk, J.J.W.; Van Rhoon, G.C.; Hornsleth, S.N.; Wust, P.; De Leeuw, A.C.C.; Schneider, C.J.; Van Ddk, J.D.P.; Van Der Zee, J.; Van Heek-Romanowski, R.; Rahman, S.A.; et al. Esho Quality Assurance Guidelines for Regional Hyperthermia. Int. J. Hyperth. 1998, 14, 125–133. [Google Scholar] [CrossRef]
- Trefna, H.D.; Schmidt, M.; Van Rhoon, G.C.; Kok, H.P.; Gordeev, S.S.; Lamprecht, U.; Marder, D.; Nadobny, J.; Ghadjar, P.; Abdel-Rahman, S.; et al. Quality assurance guidelines for interstitial hyperthermia. Int. J. Hyperth. 2019, 36, 276–293. [Google Scholar] [CrossRef] [Green Version]
- Wust, P.; Stahl, H.; Löffel, J.; Seebass, M.; Riess, H.; Felix, R. Clinical, physiological and anatomical determinants for radiofrequency hyperthermia. Int. J. Hyperth. 1995, 11, 151–167. [Google Scholar] [CrossRef]
- Wust, J.N.P. Influence of patient models and numerical methods on predicted power deposition patterns. Int. J. Hyperth. 1999, 15, 519–540. [Google Scholar] [CrossRef] [Green Version]
- Bellizzi, G.G.; Sumser, K.; VilasBoas-Ribeiro, I.; Curto, S.; Drizdal, T.; Van Rhoon, G.C.; Franckena, M.; Paulides, M.M. Standardization of patient modeling in hyperthermia simulation studies: Introducing the Erasmus Virtual Patient Repository. Int. J. Hyperth. 2020, 37, 608–616. [Google Scholar] [CrossRef]
- Schooneveldt, G.; Kok, H.P.; Bakker, A.; Geijsen, E.D.; Rasch, C.R.N.; De La Rosette, J.J.M.C.H.; Hulshof, M.C.C.M.; De Reijke, T.M.; Crezee, H. Clinical validation of a novel thermophysical bladder model designed to improve the accuracy of hyperthermia treatment planning in the pelvic region. Int. J. Hyperth. 2018, 35, 383–397. [Google Scholar] [CrossRef] [Green Version]
- Verhaart, R.F.; Fortunati, V.; Verduijn, G.M.; Van Der Lugt, A.; Van Walsum, T.; Veenland, J.F.; Paulides, M.M. The relevance of MRI for patient modeling in head and neck hyperthermia treatment planning: A comparison of CT and CT-MRI based tissue segmentation on simulated temperature. Med Phys. 2014, 41, 123302. [Google Scholar] [CrossRef]
- Paulides, M.M.; Stauffer, P.R.; Neufeld, E.; Maccarini, P.F.; Kyriakou, A.; Canters, R.A.; Diederich, C.J.; Bakker, J.F.; Van Rhoon, G.C. Simulation techniques in hyperthermia treatment planning. Int. J. Hyperth. 2013, 29, 346–357. [Google Scholar] [CrossRef]
- Canters, R.A.M.; Paulides, M.M.; Franckena, M.F.; Van Der Zee, J.; Van Rhoon, G.C. Implementation of treatment planning in the routine clinical procedure of regional hyperthermia treatment of cervical cancer: An overview and the Rotterdam experience. Int. J. Hyperth. 2012, 28, 570–581. [Google Scholar] [CrossRef]
- Canters, R.A.M.; Wust, P.; Bakker, J.; Van Rhoon, G. A literature survey on indicators for characterisation and optimisation of SAR distributions in deep hyperthermia, a plea for standardisation. Int. J. Hyperth. 2009, 25, 593–608. [Google Scholar] [CrossRef]
- Canters, R.A.M.; Franckena, M.; Paulides, M.M.; Van Rhoon, G.C. Patient positioning in deep hyperthermia: Influences of inaccuracies, signal correction possibilities and optimization potential. Phys. Med. Boil. 2009, 54, 3923–3936. [Google Scholar] [CrossRef]
- Lee, H.K.; Antell, A.G.; Perez, C.A.; Straube, W.L.; Ramachandran, G.; Myerson, R.J.; Emami, B.; Molmenti, E.P.; Buckner, A.; Lockett, M.A. Superficial hyperthermia and irradiation for recurrent breast carcinoma of the chest wall: Prognostic factors in 196 tumors. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 365–375. [Google Scholar] [CrossRef]
- Paulides, M.M.; Bakker, J.F.; Linthorst, M.; Van Der Zee, J.; Rijnen, Z.; Neufeld, E.; Pattynama, P.M.T.; Jansen, P.P.; Levendag, P.C.; Van Rhoon, G.C. The clinical feasibility of deep hyperthermia treatment in the head and neck: New challenges for positioning and temperature measurement. Phys. Med. Boil. 2010, 55, 2465–2480. [Google Scholar] [CrossRef] [Green Version]
- Van de Kamer, J.B.; Van Wieringen, N.; De Leeuw, A.A.C.; Lagendijk, J.J.W. The significance of accurate dielectric tissue data for hyperthermia. Int. J. Hyperth. 2001, 17, 123–142. [Google Scholar] [CrossRef]
- Ribeiro, I.V.; Van Holthe, N.; Van Rhoon, G.C.; Paulides, M.M. Impact of segmentation detail in hyperthermia treatment planning: Comparison between detailed and clinical tissue segmentation. In Proceedings of the EMF-Med 1st World Conference on Biomedical Applications of Electromagnetic Fields (EMF-Med), Split, Croatia, 10–13 September 2018. [Google Scholar]
- Hornsleth, S.N.; Mella, O.; Dahl, O. A new CT segmentation algorithm for finite difference based treatment planning systems. Hyperthermic Oncol. 1996, 2, 521–523. [Google Scholar]
- Gabriel, C.; Gabriel, S.; Corthout, E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Boil. 1996, 41, 2231–2249. [Google Scholar] [CrossRef] [Green Version]
- Christ, A.; Kainz, W.; Hahn, E.G.; Honegger, K.; Zefferer, M.; Neufeld, E.; Rascher, W.; Janka, R.; Bautz, W.; Chen, J.; et al. The Virtual Family—Development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys. Med. Boil. 2009, 55, N23–N38. [Google Scholar] [CrossRef]
- Gosselin, M.-C.; Neufeld, E.; Moser, H.; Huber, E.; Farcito, S.; Gerber, L.; Jedensjö, M.; Hilber, I.; Di Gennaro, F.; Lloyd, B.; et al. Development of a new generation of high-resolution anatomical models for medical device evaluation: The Virtual Population 3.0. Phys. Med. Boil. 2014, 59, 5287–5303. [Google Scholar] [CrossRef] [PubMed]
- Rijnen, Z.; Bakker, J.F.; Canters, R.A.; Togni, P.; Verduijn, G.M.; Levendag, P.C.; Van Rhoon, G.C.; Paulides, M.M. Clinical integration of software tool VEDO for adaptive and quantitative application of phased array hyperthermia in the head and neck. Int. J. Hyperth. 2013, 29, 181–193. [Google Scholar] [CrossRef]
- McIntosh, R.L.; Anderson, V. A Comprehensive Tissue Properties Database Provided for the Thermal Assessment of A Human at Rest. Biophys. Rev. Lett. 2010, 5, 129–151. [Google Scholar] [CrossRef]
- Hasgall, P.A.; Neufeld, E.; Gosselin, M.C.; Klingenbck, A.; Kuster, N.K. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues. Version 4.0. 15 May 2018. [Google Scholar] [CrossRef]
- Franckena, M.; Lutgens, L.C.; Koper, P.C.; Kleynen, C.E.; Van Der Steen-Banasik, E.M.; Jobsen, J.J.; Leer, J.W.; Creutzberg, C.L.; Dielwart, M.F.; Van Norden, Y.; et al. Radiotherapy and Hyperthermia for Treatment of Primary Locally Advanced Cervix Cancer: Results in 378 Patients. Int. J. Radiat. Oncol. 2009, 73, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Rau, B.; Wust, P.; Tilly, W.; Gellermann, J.; Harder, C.; Riess, H.; Budach, V.; Félix, R.; Schlag, P.M. Preoperative radiochemotherapy in locally advanced or recurrent rectal cancer: Regional radiofrequency hyperthermia correlates with clinical parameters. Int. J. Radiat. Oncol. 2000, 48, 381–391. [Google Scholar] [CrossRef]
- Sreenivasa, G.; Hildebrandt, B.; Kümmel, S.; Jungnickel, K.; Cho, C.H.; Tilly, W.; Böhmer, D.; Budach, V.; Felix, R.; Wust, P. Radiochemotherapy combined with regional pelvic hyperthermia induces high response and resectability rates in patients with nonresectable cervical cancer ≥FIGO IIB “bulky”. Int. J. Radiat. Oncol. 2006, 66, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Dinges, S.; Harder, C.; Wurm, R.; Buchali, A.; Blohmer, J.; Gellermann, J.; Wust, P.; Randow, H.; Budach, V. Combined treatment of inoperable carcinomas of the uterine cervix with radiotherapy and regional hyperthermia. Results of a phase II trial. Strahlenther. Onkol. 1998, 174, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Tilly, W.; Gellermann, J.; Graf, R.; Hildebrandt, B.; Weißbach, L.; Budach, V.; Felix, R.; Wust, P. Regional hyperthermia in conjunction with definitive radiotherapy against recurrent or locally advanced prostate cancer T3 pNO MO. Strahlenther. Onkol. 2005, 181, 35–41. [Google Scholar] [CrossRef]
- A Yoon, M.; Hong, S.-J.; Ku, M.C.; Kang, C.H.; Ahn, K.-S.; Kim, B.H. Multiparametric MR Imaging of Age-related Changes in Healthy Thigh Muscles. Radiology 2018, 287, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Juang, T.; Stauffer, P.R.; Craciunescu, O.A.; Maccarini, P.F.; Yuan, Y.; Das, S.K.; Dewhirst, M.W.; Inman, B.A.; Vujaskovic, Z. Thermal dosimetry characteristics of deep regional heating of non-muscle invasive bladder cancer. Int. J. Hyperth. 2014, 30, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Zweije, R.; Kok, H.P.; Bakker, A.; Bel, A.; Crezee, J. Technical and Clinical Evaluation of the ALBA-4D 70MHz Loco-Regional Hyperthermia System. In Proceedings of the 2018 48th European Microwave Conference (EuMC), Madrid, Spain, 23–27 September 2018; Volume 2, pp. 328–331. [Google Scholar] [CrossRef]
- Gellermann, J.; Wlodarczyk, W.; Ganter, H.; Nadobny, J.; Fähling, H.; Seebass, M.; Felix, R.; Wust, P. A practical approach to thermography in a hyperthermia/magnetic resonance hybrid system: Validation in a heterogeneous phantom. Int. J. Radiat. Oncol. 2005, 61, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Curto, S.; Aklan, B.; Mulder, H.T.; Mils, O.; Schmidt, M.; Lamprecht, U.; Peller, M.; Wessalowski, R.; Lindner, L.H.; Fietkau, R.; et al. Quantitative, Multi-institutional Evaluation of MR Thermometry Accuracy for Deep-Pelvic MR-Hyperthermia Systems Operating in Multi-vendor MR-systems Using a New Anthropomorphic Phantom. Cancers 2019, 11, 1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, J.; Paulides, M.M.; Neufeld, E.; Christ, A.; Kuster, N.; Van Rhoon, G.C. Children and adults exposed to electromagnetic fields at the ICNIRP reference levels: Theoretical assessment of the induced peak temperature increase. Phys. Med. Boil. 2011, 56, 4967–4989. [Google Scholar] [CrossRef] [PubMed]






| Segmentation List Name | Delineated Tissues |
|---|---|
| Detailed | 80 tissues and GTV |
| Clinical | fat, muscle, cortical bone, and GTV |
| Bone-type | fat, muscle, cortical bone, GTV, bone marrow, and cancellous bone |
| Bladder | fat, muscle, cortical bone, GTV, urine, and bladder wall |
| Intestine | fat, muscle, cortical bone, GTV, small intestine wall, small intestine lumen, large intestine wall, large intestine lumen |
| Bladder and intestine | fat, muscle, cortical bone, GTV, urine, bladder wall, small intestine wall, small intestine lumen, large intestine wall, and large intestine lumen |
| Combined | fat, muscle, cortical bone, GTV, bone marrow, cancellous bone, urine, bladder wall, small intestine wall, small intestine lumen, large intestine wall, and large intestine lumen |
| Tissue | |||
|---|---|---|---|
| Applicator shell | 2.0 | 0.004 | 1180 |
| Water (water bolus) | 80.9 | 0.002 | 1000 |
| Fat | 12.7 | 0.007 | 911 |
| Muscle | 66.0 | 0.708 | 1090 |
| Bone (cortical) | 15.3 | 0.006 | 1908 |
| Bone (marrow) | 14.3 | 0.159 | 1029 |
| Bone (cancellous) | 27.6 | 0.173 | 1178 |
| Small intestine wall | 96.5 | 1.660 | 1030 |
| Small intestine lumen | 80.0 | 2.000 | 1000 |
| Large intestine wall | 81.8 | 0.680 | 1088 |
| Large intestine lumen | 66.0 | 0.708 | 1090 |
| Bladder wall | 22.7 | 0.294 | 1086 |
| Urine | 49.9 | 1.750 | 1024 |
| Tumor (GTV) | 70.0 | 0.750 | 1050 |
| Segmentation | Cervix Cancer | Rectum Cancer | ||||
|---|---|---|---|---|---|---|
| Billie | Ella | Yoon-Sun | Billie | Ella | Yoon-Sun | |
| Clinic | 22.8 ± 7.2 | 15.0 ± 3.9 | 7.0 ± 0.6 | 9.9 ± 3.1 | 18.7 ± 1.3 | 8.2 ± 1.6 |
| Bone-type | 21.1 ± 5.6 | 11.3 ± 5.8 | 5.6 ± 0.3 | 10.3 ± 2.0 | 18.1 ± 4.1 | 5.8 ± 0.1 |
| Bladder | 23.8 ± 7.4 | 20.8 ± 9.6 | 10.2 ± 1.3 | 13.0 ± 2.6 | 16.7 ± 2.7 | 7.4 ± 1.0 |
| Intestine | 5.3 ± 2.4 | 12.2 ± 5.2 | 11.8 ± 4.3 | 5.8 ± 2.2 | 13.2 ± 2.8 | 5.2 ± 0.3 |
| Blad. and Int. | 8.1 ± 3.5 | 10.2 ± 3.2 | 18.0 ± 7.7 | 14.0 ± 6.6 | 15.1 ± 3.0 | 5.9 ± 1.1 |
| Combined | 3.4 ± 0.1 | 7.8 ± 3.5 | 8.2 ± 3.0 | 0.7 ± 0.3 | 14.5 ± 3.0 | 4.7 ± 0.9 |
| Segmentation | Prostate Cancer | Rectum Cancer | ||
|---|---|---|---|---|
| Louis | Duke | Louis | Duke | |
| Clinic | 20.6 ± 0.6 | 4.4 ± 1.3 | 5.0 ± 1.6 | 3.1 ± 0.5 |
| Bone-type | 21.6 ± 2.4 | 4.7 ± 1.3 | 3.7 ± 1.2 | 7.6 ± 2.4 |
| Bladder | 3.6 ± 0.8 | 2.2 ± 0.2 | 0.7 ± 0.2 | 2.9 ± 2.3 |
| Intestine | 22.1 ± 2.0 | 6.2 ± 1.4 | 3.2 ± 0.8 | 2.6 ± 1.2 |
| Blad. and Int. | 14.2 ± 3.9 | 2.4 ± 0.8 | 1.2 ± 0.6 | 1.8 ± 0.9 |
| Combined | 8.0 ± 3.3 | 2.1 ± 0.9 | 1.3 ± 0.6 | 1.9 ± 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
VilasBoas-Ribeiro, I.; van Rhoon, G.C.; Drizdal, T.; Franckena, M.; Paulides, M.M. Impact of Number of Segmented Tissues on SAR Prediction Accuracy in Deep Pelvic Hyperthermia Treatment Planning. Cancers 2020, 12, 2646. https://doi.org/10.3390/cancers12092646
VilasBoas-Ribeiro I, van Rhoon GC, Drizdal T, Franckena M, Paulides MM. Impact of Number of Segmented Tissues on SAR Prediction Accuracy in Deep Pelvic Hyperthermia Treatment Planning. Cancers. 2020; 12(9):2646. https://doi.org/10.3390/cancers12092646
Chicago/Turabian StyleVilasBoas-Ribeiro, Iva, Gerard C. van Rhoon, Tomas Drizdal, Martine Franckena, and Margarethus M. Paulides. 2020. "Impact of Number of Segmented Tissues on SAR Prediction Accuracy in Deep Pelvic Hyperthermia Treatment Planning" Cancers 12, no. 9: 2646. https://doi.org/10.3390/cancers12092646
APA StyleVilasBoas-Ribeiro, I., van Rhoon, G. C., Drizdal, T., Franckena, M., & Paulides, M. M. (2020). Impact of Number of Segmented Tissues on SAR Prediction Accuracy in Deep Pelvic Hyperthermia Treatment Planning. Cancers, 12(9), 2646. https://doi.org/10.3390/cancers12092646