High Serum PD-L1 Levels Are Associated with Poor Survival in Urothelial Cancer Patients Treated with Chemotherapy and Immune Checkpoint Inhibitor Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
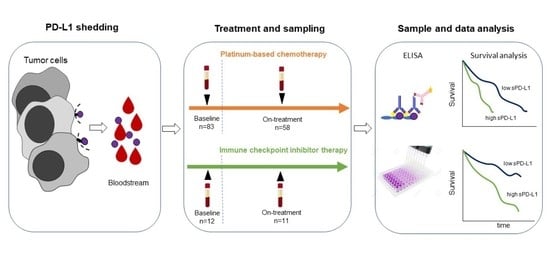
2. Materials and Methods
2.1. Patients
2.2. Serum PD-L1 Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Tissue PD-L1 Immunohistochemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Background
3.2. Correlation of Serum PD-L1 Concentrations with Clinicopathological Parameters and Survival
3.3. Serum PD-L1 Level Changes during Chemotherapy
3.4. Serum PD-L1 Level Changes during ICI Therapy
3.5. Tissue PD-L1 Expression and Its Corrrelation with Serum PD-L1 Levels
3.6. Correlation Between Serum PD-L1 and MMP-7 Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Stein, J.P.; Skinner, D.G. Radical cystectomy for invasive bladder cancer: Long-term results of a standard procedure. World J. Urol. 2006, 24, 296–304. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Trinh, Q.-D.; Seisen, T.; Vetterlein, M.W.; Sammon, J.; Preston, M.A.; Lipsitz, S.R.; Bellmunt, J.; Menon, M.; Choueiri, T.K.; et al. Effectiveness of Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer in the Current Real World Setting in the USA. Eur. Urol. Oncol. 2018, 1, 83–90. [Google Scholar] [CrossRef]
- Von Der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, With Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients with Bladder Cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef] [PubMed]
- E Rosenberg, J.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Ning, Y.; Suzman, D.; Maher, V.E.; Zhang, L.; Tang, S.; Ricks, T.; Palmby, T.; Fu, W.; Liu, Q.; Goldberg, K.B.; et al. FDA Approval Summary: Atezolizumab for the Treatment of Patients with Progressive Advanced Urothelial Carcinoma after Platinum-Containing Chemotherapy. Oncologist 2017, 22, 743–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzman, D.L.; Agrawal, S.; Ning, Y.; Maher, V.E.; Fernandes, L.L.; Karuri, S.; Tang, S.; Sridhara, R.; Schroeder, J.; Goldberg, K.B.; et al. FDA Approval Summary: Atezolizumab or Pembrolizumab for the Treatment of Patients with Advanced Urothelial Carcinoma Ineligible for Cisplatin-Containing Chemotherapy. Oncologist 2019, 24, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakanishi, J.; Wada, Y.; Matsumoto, K.; Azuma, M.; Kikuchi, K.; Ueda, S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 2007, 56, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Dezutter-Dambuyant, C.; Durand, I.; Alberti, L.; Bendriss-Vermare, N.; Valladeau-Guilemond, J.; Duc, A.; Magron, A.; Morel, A.-P.; Sisirak, V.; Rodriguez, C.; et al. A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage. OncoImmunology 2016, 5, e1091146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantini, A.; Julie, C.; Dumenil, C.; Hélias-Rodzewicz, Z.; Tisserand, J.; Dumoulin, J.; Giraud, V.; Labrune, S.; Chinet, T.; Emile, J.-F.; et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. OncoImmunology 2018, 7, e1452581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, T.; Kamai, T.; Masuda, A.; Nukui, A.; Abe, H.; Arai, K.; Yoshida, K. Higher preoperative serum levels of PD -L1 and B7-H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF -targeted therapy and unfavorable prognosis of renal cell carcinoma. Cancer Med. 2016, 5, 1810–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushlinskii, N.; Gershtein, E.S.; Morozov, A.A.; Goryacheva, I.O.; Filipenko, M.L.; Alferov, A.A.; Bezhanova, S.D.; Bazaev, V.V.; Kazantseva, I.A. Soluble Ligand of the Immune Checkpoint Receptor (sPD-L1) in Blood Serum of Patients with Renal Cell Carcinoma. Bull. Exp. Biol. Med. 2019, 166, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T.; Becker, M.; Dorp, F.V.; Gethmann, C.; Tötsch, M.; Bánkfalvi, Á.; Schmid, K.W.; Romics, I.; Rübben, H.; Ergün, S. Matrix metalloproteinase-7 as a marker of metastasis and predictor of poor survival in bladder cancer. Cancer Sci. 2010, 101, 1300–1308. [Google Scholar] [CrossRef]
- Szarvas, T.; Singer, B.B.; Becker, M.; Dorp, F.V.; Jäger, T.; Szendrői, A.; Riesz, P.; Romics, I.; Rübben, H.; Ergün, S. Urinary matrix metalloproteinase-7 level is associated with the presence of metastasis in bladder cancer. BJU Int. 2010, 107, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T.; Hoffmann, M.J.; Olah, C.; Szekely, E.; Kiss, A.; Hess, J.; Tschirdewahn, S.; Hadaschik, B.; Grotheer, V.; Nyirady, P.; et al. MMP-7 Serum and Tissue Levels Are Associated with Poor Survival in Platinum-Treated Bladder Cancer Patients. Diagnostics 2020, 11, 48. [Google Scholar] [CrossRef]
- Hira-Miyazawa, M.; Nakamura, H.; Hirai, M.; Kobayashi, Y.; Kitahara, H.; Bou-Gharios, G.; Kawashiri, S. Regulation of programmed-death ligand in the human head and neck squamous cell carcinoma microenvironment is mediated through matrix metalloproteinase-mediated proteolytic cleavage. Int. J. Oncol. 2017, 52, 379–388. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Liu, J.; Pan, T.; Zhou, T.; Liu, Z.; Tan, R.; Wang, X.; Tian, L.; Chen, E.; et al. sPD-L1 Expression is Associated with Immunosuppression and Infectious Complications in Patients with Acute Pancreatitis. Scand. J. Immunol. 2017, 86, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Szarvas, T.; Oláh, C.; Riesz, P.; Géczi, L.; Nyirády, P. A húgyhólyag urothelialis daganatainak molekuláris alcsoportbeosztása és annak klinikai vonatkozásai. Orvosi Hetil. 2019, 160, 1647–1654. [Google Scholar] [CrossRef]
- Als, A.B.; Dyrskjøt, L.; Von Der Maase, H.; Koed, K.; Mansilla, F.; Toldbod, H.E.; Jensen, J.L.; Ulhøi, B.P.; Sengeløv, L.; Jensen, K.M.; et al. Emmprin and Survivin Predict Response and Survival following Cisplatin-Containing Chemotherapy in Patients with Advanced Bladder Cancer. Clin. Cancer Res. 2007, 13, 4407–4414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groenendijk, F.H.; de Jong, J.; van de Putte, E.E.F.; Michaut, M.; Schlicker, A.; Peters, D.; Velds, A.; Nieuwland, M.; Heuvel, M.M.V.D.; Kerkhoven, R.M.; et al. ERBB2 Mutations Characterize a Subgroup of Muscle-invasive Bladder Cancers with Excellent Response to Neoadjuvant Chemotherapy. Eur. Urol. 2016, 69, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Pichler, R.; Heidegger, I.; Fritz, J.; Danzl, M.; Sprung, S.; Zelger, B.; Brunner, A.; Pircher, A. PD-L1 expression in bladder cancer and metastasis and its influence on oncologic outcome after cystectomy. Oncotarget 2017, 8, 66849–66864. [Google Scholar] [CrossRef] [Green Version]
- Shigemori, T.; Toiyama, Y.; Okugawa, Y.; Yamamoto, A.; Yin, C.; Narumi, A.; Ichikawa, T.; Ide, S.; Shimura, T.; Fujikawa, H.; et al. Soluble PD-L1 Expression in Circulation as a Predictive Marker for Recurrence and Prognosis in Gastric Cancer: Direct Comparison of the Clinical Burden Between Tissue and Serum PD-L1 Expression. Ann. Surg. Oncol. 2018, 26, 876–883. [Google Scholar] [CrossRef]
- Tominaga, T.; Akiyoshi, T.; Yamamoto, N.; Taguchi, S.; Mori, S.; Nagasaki, T.; Fukunaga, Y.; Ueno, M. Clinical significance of soluble programmed cell death-1 and soluble programmed cell death-ligand 1 in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. PLoS ONE 2019, 14, e0212978. [Google Scholar] [CrossRef] [Green Version]
- Rossille, D.; Gressier, M.; Damotte, D.; Maucort-Boulch, D.; Pangault, C.; Semana, G.; Le Gouill, S.; Haioun, C.; Tarte, K.; Lamy, T.; et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: Results from a French multicenter clinical trial. Leukemia 2014, 28, 2367–2375. [Google Scholar] [CrossRef]
- Ruf, M.; Moch, H.; Schraml, P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int. J. Cancer 2016, 139, 396–403. [Google Scholar] [CrossRef] [Green Version]
- E Aguirre, J.; Beswick, E.J.; Grim, C.; Uribe, G.; Tafoya, M.; Palma, G.C.; Samedi, V.; McKee, R.; Villeger, R.; Fofanov, Y.; et al. Matrix metalloproteinases cleave membrane-bound PD-L1 on CD90+ (myo-)fibroblasts in Crohn’s disease and regulate Th1/Th17 cell responses. Int. Immunol. 2020, 32, 57–68. [Google Scholar] [CrossRef]
- Zhu, X.; Lang, J. Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer. Oncotarget 2017, 8, 97671–97682. [Google Scholar] [CrossRef] [Green Version]
- Sarfaty, M.; Rosenberg, J.E. Antibody-Drug Conjugates in Urothelial Carcinomas. Curr. Oncol. Rep. 2020, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, E.; Fujita, K.; Pena, M.R.; Taheri, D.; Banno, E.; Kato, T.; Hatano, K.; Kawashima, A.; Ujike, T.; Uemura, M.; et al. Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2020, 21, 5390. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Kockx, M.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Szabados, B.; Pous, A.F.; Gravis, G.; Herranz, U.A.; et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 2019, 25, 1706–1714. [Google Scholar] [CrossRef] [PubMed]

| Variables | Whole Chemo Cohort | ICI Cohort |
|---|---|---|
| n (%) | n (%) | |
| Total number of patients | 83 | 12 |
| Age at baseline median (range) | 67 (41–81) | 69 (63–77) |
| Gender | ||
| male | 64 (77) | 10 (83) |
| female | 19 (33) | 2 (17) |
| ECOG PS at enrollment | ||
| 0 | 48 (58) | 10 (84) |
| 1 | 32 (37) | 1 (8) |
| 2 | 3 (3) | 1 (8) |
| Cystectomy data | ||
| Cystectomy performed | 59 (71) | 8 (67) |
| pT0 | 3 (5) | 1 (8) |
| pT1 | 2 (3) | 0 (0) |
| pT2 | 11 (19) | 1 (8) |
| pT3 | 30 (51) | 5 (42) |
| pT4 | 12 (20) | 1 (8) |
| n.a. | 1 | 4 (33) |
| R | 10 (17) | 0 (0) |
| LN metastasis at RC | 30 (51) | 5 (42) |
| Chemo baseline data | ||
| LN at baseline | 36 (43) | 4 (33) |
| Distant metastasis at baseline | 12 (15) | 7 (58) |
| Soft tissue lesions (lung/liver) | 9 (11) | 5 (42) |
| Bone metastasis | 3 (3) | 2 (17) |
| Setting of chemotherapy | ||
| neoadjuvant | 22 (27) | - |
| adjuvant | 33 (40) | - |
| palliative | 28 (33) | - |
| Chemotherapy regimen | ||
| Gem/Cis | 61 (73) | - |
| Gem/Carbo | 22 (27) | - |
| Atezolizumab | - | 11 (92) |
| Pembrolizumab | - | 1 (8) |
| Number of cycles median (range) | 3 (1–9) | 5 (2–17) |
| single (only one series) | 9 | 0 |
| Number of patients died (%) | 45 (54) | 4 (33) |
| Follow-up time in months median (range) | 14 (1–80) | 17 (6–31) |
| PD-L1 serum levels | ||
| PD-L1 median (range) baseline [pg/mL] | 83.0 (0.0–781) | 90.0 (25.3–169.0) |
| PD-L1 median (range) 2–3 cycle [pg/mL] | 78.5 (0.0–273.1) | 2316 (42.5–3818) |
| Variables | All Patients | Whole Chemo Cohort | ICI Cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Median (Range) | p | n | Median (Range) | p | n | Median (Range) | p | ||
| Age | ≤65 | 38 | 84.4 (0.0–780.9) | 0.566 | 35 | 82.2 (0.0–780.9) | 0.430 | 3 | 86.6 (59.0–169.0) | - |
| >65 | 57 | 84.9 (25.3–243.1) | 48 | 84.0 (35.8–243.1) | 9 | 93.3 (25.3–145.0) | ||||
| Gender | Male | 74 | 86.1 (0.0–780.9) | 0.950 | 64 | 84.0 (0.0–780.9) | 0.435 | 10 | 94.2 (59.0–169.0) | - |
| Female | 10 | 82.2 (25.3–194.9) | 19 | 82.2 (40.5–194.9) | 2 | 26.1 (25.3–26.89) | ||||
| ECOG | 0 | 58 | 80.8 (0.0–169.0) | 0.022 | 48 | 80.4 (0.0–158.5) | 0.007 | 10 | 91.9 (26.9–169.0) | - |
| 1–2 | 37 | 95.9 (25.3–780.9) | 35 | 96.2 (35.8–780.9) | 2 | 59.3 (25.3–93.3) | ||||
| Stage at cystectomy | Not performed | 28 | 24 | 4 | ||||||
| n.a. | 1 | 1 | 0 | |||||||
| pT0 | 4 | 92.2 (46.0–111.4) | 0.792 | 3 | 97.7 (46.0–111.0) | 0.858 | 1 | 86.6 | - | |
| pT1 | 2 | 135.2 (75.6–194.9) | 2 | 135.2 (75.6–194.9) | 0 | - | ||||
| pT2 | 12 | 78.4 (26.9–154.9) | 11 | 80.7 (32.4–154.9) | 1 | 26.9 | ||||
| pT3 | 35 | 81.5 (25.8–324.0) | 30 | 80.4 (25.8–324.0) | 5 | 99.1 (59.0–169.0) | ||||
| pT4 | 13 | 88.7 (0.0–158.5) | 12 | 84.4 (0.0–158.5) | 1 | 93.3 | ||||
| pT1–pT2 | 18 | 83.7 (26.9–194.9) | 0.259 | 16 | 84.7 (32.4–194.9) | 0.741 | 2 | 56.8 (26.9–86.6) | - | |
| pT3–pT4 | 48 | 85.1 (0.0–324.0) | 42 | 80.4 (0.0–324.0) | 6 | 97.2 (59.0–169.0) | ||||
| LN/M status | N0/M0 | 40 | 88.6 (25.8–324.0) | 0.277 | 38 | 87.0 (38.5–324.0) | 0.209 | 2 | 87.1 (80.9–93.3) | - |
| N+ or M+ | 55 | 85.1 (0.0–158.8) | 45 | 80.7 (0.0–780.9) | 10 | 90.9 (25.3–169.0) | ||||
| Variables | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| n | HR | 95% CI | p | HR | 95% CI | p | ||
| Ag | ≤65 | 35 | ref. | - | - | - | ||
| >65 | 48 | 1.297 | 0.716–2.351 | 0.391 | - | - | - | |
| Gender | Male | 64 | ref. | - | - | - | ||
| Female | 19 | 0.815 | 0.392–1.694 | 0.584 | - | - | - | |
| ECOG | 0 | 48 | ref. | ref. | ||||
| 1–2 | 35 | 2.615 | 1.381–4.950 | 0.003 | 3.692 | 1.870–7.287 | <0.001 | |
| Stage at cystectomy * | T0–T2 | 16 | ref. | - | - | - | ||
| T3–T4 | 42 | 1.135 | 0.483–2.664 | 0.772 | - | - | - | |
| LN/M status * | N0/M0 | 38 | ref. | ref. | ||||
| N+ or M+ | 45 | 1.914 | 1.017–3.601 | 0.044 | 2.904 | 1.502–5.615 | 0.002 | |
| PD-L1 serum cc./upper 25% | <103 pg/mL | 63 | ref. | ref. | ||||
| ≥103 pg/mL | 20 | 1.381 | 1.120–1.703 | 0.002 | 1.41 | 1.135–1.752 | 0.002 | |
| PD-L1 serum cc. continuous | - | 83 | 1.002 | 0.999–1.004 | 0.126 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krafft, U.; Olah, C.; Reis, H.; Kesch, C.; Darr, C.; Grünwald, V.; Tschirdewahn, S.; Hadaschik, B.; Horvath, O.; Kenessey, I.; et al. High Serum PD-L1 Levels Are Associated with Poor Survival in Urothelial Cancer Patients Treated with Chemotherapy and Immune Checkpoint Inhibitor Therapy. Cancers 2021, 13, 2548. https://doi.org/10.3390/cancers13112548
Krafft U, Olah C, Reis H, Kesch C, Darr C, Grünwald V, Tschirdewahn S, Hadaschik B, Horvath O, Kenessey I, et al. High Serum PD-L1 Levels Are Associated with Poor Survival in Urothelial Cancer Patients Treated with Chemotherapy and Immune Checkpoint Inhibitor Therapy. Cancers. 2021; 13(11):2548. https://doi.org/10.3390/cancers13112548
Chicago/Turabian StyleKrafft, Ulrich, Csilla Olah, Henning Reis, Claudia Kesch, Christopher Darr, Viktor Grünwald, Stephan Tschirdewahn, Boris Hadaschik, Orsolya Horvath, Istvan Kenessey, and et al. 2021. "High Serum PD-L1 Levels Are Associated with Poor Survival in Urothelial Cancer Patients Treated with Chemotherapy and Immune Checkpoint Inhibitor Therapy" Cancers 13, no. 11: 2548. https://doi.org/10.3390/cancers13112548
APA StyleKrafft, U., Olah, C., Reis, H., Kesch, C., Darr, C., Grünwald, V., Tschirdewahn, S., Hadaschik, B., Horvath, O., Kenessey, I., Nyirady, P., Varadi, M., Modos, O., Csizmarik, A., & Szarvas, T. (2021). High Serum PD-L1 Levels Are Associated with Poor Survival in Urothelial Cancer Patients Treated with Chemotherapy and Immune Checkpoint Inhibitor Therapy. Cancers, 13(11), 2548. https://doi.org/10.3390/cancers13112548