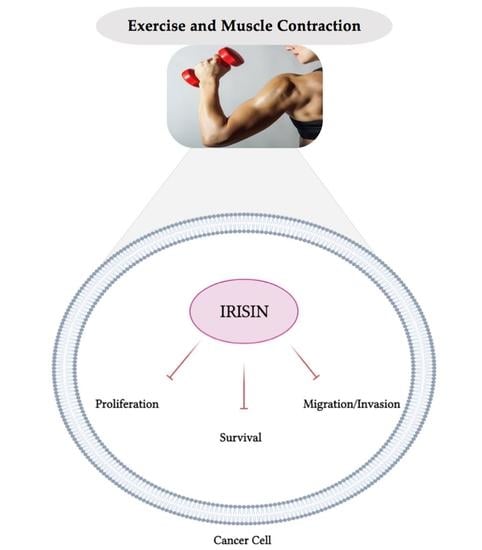
Current Evidence of the Role of the Myokine Irisin in Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Irisin
2.1. Irisin Structure and Synthesis
2.2. Irisin Blood Levels, Clearance and Tissue Distribution
2.3. Irisin receptor and Mechanism of Action in Target Tissues
3. Role of Irisin in Cancer
3.1. Role of Irisin in Cancer: In Vitro Evidence
3.2. Role of Irisin in Cancer: In Vivo Evidence
3.2.1. Animal Models of Cancer
3.2.2. Human Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, caac.21660. [Google Scholar] [CrossRef]
- McCormick, P.J. Cancer Tsunami: Emerging Trends, Economic Burden, AndPerioperative Implications. Curr. Anesthesiol. Rep. 2018, 8, 348–354. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell. 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell. Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Shaw, R.J.; Cantley, L.C. Ras, PI (3) K and MTOR Signalling Controls Tumour Cell Growth. Nature 2006, 441, 424–430. [Google Scholar] [CrossRef]
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT Signaling Pathway and Cancer: An Updated Review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef]
- Mossmann, D.; Park, S.; Hall, M.N. MTOR Signalling and Cellular Metabolism Are Mutual Determinants in Cancer. Nat. Rev. Cancer 2018, 18, 744–757. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP Kinase Signalling Pathways in Cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [Green Version]
- Haglund, K.; Rusten, T.E.; Stenmark, H. Aberrant Receptor Signaling and Trafficking as Mechanisms in Oncogenesis. Crit. Rev. Oncog. 2007, 13, 39–74. [Google Scholar] [CrossRef]
- Hobbs, G.A.; Der, C.J.; Rossman, K.L. RAS Isoforms and Mutations in Cancer at a Glance. J. Cell Sci. 2016, 129, 1287–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; George, B.; Campbell, M.R.; Verma, N.; Paul, A.M.; Melo-Alvim, C.; Ribeiro, L.; Pillai, M.R.; da Costa, L.M.; Moasser, M.M. HER Family in Cancer Progression: From Discovery to 2020 and Beyond. Adv. Cancer Res. 2020, 147, 109–160. [Google Scholar] [CrossRef] [PubMed]
- Page, C.; Lin, H.J.; Jin, Y.; Castle, V.P.; Nunez, G.; Huang, M.; Lin, J. Overexpression of Akt/AKT Can Modulate Chemotherapy-Induced Apoptosis. Anticancer Res. 2000, 20, 407–416. [Google Scholar]
- Wang, Q.; Chen, X.; Hay, N. Akt as a Target for Cancer Therapy: More Is Not Always Better (Lessons from Studies in Mice). Br. J. Cancer 2017, 117, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Pixu, L.; Hailing, C.; Roberts, T.M.; Zhao, J.J. Targeting the Phosphoinositide 3-Kinase Pathway in Cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.H.; Yap, T.A.; Yan, L.; Cunningham, D. Targeting the PI3K-AKT-MTOR Signaling Network in Cancer. Chin. J. Cancer 2013, 32, 253–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuurbiers, O.C.J.; Kaanders, J.H.A.M.; van der Heijden, H.F.M.; Dekhuijzen, R.P.N.; Oyen, W.J.G.; Bussink, J. The PI3-K/AKT-Pathway and Radiation Resistance Mechanisms in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2009, 4, 761–767. [Google Scholar] [CrossRef]
- Xia, S.; Zhao, Y.; Yu, S.; Zhang, M. Activated PI3K/Akt/COX-2 Pathway Induces Resistance to Radiation in Human Cervical Cancer HeLa Cells. Cancer Biother. Radiopharm. 2010, 25, 317–323. [Google Scholar] [CrossRef]
- Kim, J.; Guan, K.-L. MTOR as a Central Hub of Nutrient Signalling and Cell Growth. Nat. Cell. Biol. 2019, 21, 63–71. [Google Scholar] [CrossRef]
- Guertin, D.A.; Sabatini, D.M. Defining the Role of MTOR in Cancer. Cancer Cell. 2007, 12, 9–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pópulo, H.; Lopes, J.M.; Soares, P. The MTOR Signalling Pathway in Human Cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, Y.; Huang, S. Updates of MTOR Inhibitors. Anticancer Agents Med. Chem. 2010, 10, 571–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitot, H.C. The Molecular Biology of Carcinogenesis. Cancer 1993, 72, 962–970. [Google Scholar] [CrossRef]
- Thune, I.; Lund, E. The Influence of Physical Activity on Lung-Cancer Risk: A Prospective Study of 81,516 Men and Women. Int. J. Cancer 1997, 70, 57–62. [Google Scholar] [CrossRef]
- Thune, I.; Furberg, A.-S. Physical Activity and Cancer Risk: Dose-Response and Cancer, All Sites and Site-Specific. Med. Sci. Sports Exerc. 2001, 33, S530. [Google Scholar] [CrossRef] [PubMed]
- Willer, A. Reduction of the Individual Cancer Risk by Physical Exercise. Onkologie 2003, 26, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.D.; Chen, W.Y.; Feskanich, D.; Kroenke, C.H.; Colditz, G.A. Physical Activity and Survival after Breast Cancer Diagnosis. JAMA 2005, 293, 2479–2486. [Google Scholar] [CrossRef] [Green Version]
- Friedenreich, C.M.; Neilson, H.K.; Lynch, B.M. State of the Epidemiological Evidence on Physical Activity and Cancer Prevention. Eur. J. Cancer 2010, 46, 2593–2604. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, F.; Li, D.; Wang, F.; Zhu, L.; Chen, W.; Ge, J.; An, R.; Zhao, Y. Does Physical Activity Reduce the Risk of Prostate Cancer? A Systematic Review and Meta-Analysis. Eur. Urol. 2011, 16, 1029–1044. [Google Scholar] [CrossRef]
- Kruk, J.; Czerniak, U. Physical Activity and Its Relation to Cancer Risk: Updating the Evidence. Asian Pac. J. Cancer Prev. APJCP 2013, 14, 3993–4003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, P.T. Significantly Greater Reduction in Breast Cancer Mortality from Post-Diagnosis Running than Walking. Int. J. Cancer 2014, 135, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.; Dos Santos, G.; Guillas, G.; Bertsch, J.; Duclos, M.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; Mesrine, S. Recent Recreational Physical Activity and Breast Cancer Risk in Postmenopausal Women in the E3N Cohort. Cancer Epidemiol. Prev. Biomark. 2014, 23, 1893–1902. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.C.; Lee, I.-M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; de Gonzalez, A.B.; Hartge, P.; et al. Leisure-Time Physical Activity and Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Shaw, E.; Neilson, H.K.; Brenner, D.R. Epidemiology and Biology of Physical Activity and Cancer Recurrence. J. Mol. Med. Berl. Ger. 2017, 95, 1029–1041. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Rogers, C.J. Physical Activity and Breast Cancer Prevention: Possible Role of Immune Mediators. Front. Nutr. 2020, 7, 557997. [Google Scholar] [CrossRef]
- Cannioto, R.A.; Hutson, A.; Dighe, S.; McCann, W.; McCann, S.E.; Zirpoli, G.R.; Barlow, W.; Kelly, K.M.; DeNysschen, C.A.; Hershman, D.L.; et al. Physical Activity Before, During, and After Chemotherapy for High-Risk Breast Cancer: Relationships with Survival. J. Natl. Cancer Inst. 2021, 113, 54–63. [Google Scholar] [CrossRef]
- Raschke, S.; Eckel, J. Adipo-Myokines: Two Sides of the Same Coin--Mediators of Inflammation and Mediators of Exercise. Mediat. Inflamm. 2013, 2013, 320724. [Google Scholar] [CrossRef] [PubMed]
- So, B.; Kim, H.-J.; Kim, J.; Song, W. Exercise-Induced Myokines in Health and Metabolic Diseases. Integr. Med. Res. 2014, 3, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Di Raimondo, D.; Miceli, G.; Musiari, G.; Tuttolomondo, A.; Pinto, A. New Insights about the Putative Role of Myokines in the Context of Cardiac Rehabilitation and Secondary Cardiovascular Prevention. Ann. Transl. Med. 2017, 5, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydin, S. Is Irisin a Decisive Protein in Cancer Cachexia and Death of Cancer Cells? Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3727–3729. [Google Scholar] [PubMed]
- Thomas, R.J.; Kenfield, S.A.; Jimenez, A. Exercise-Induced Biochemical Changes and Their Potential Influence on Cancer: A Scientific Review. Br. J. Sports Med. 2017, 51, 640–644. [Google Scholar] [CrossRef]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Med. Kaunas Lith. 2019, 55, 485. [Google Scholar] [CrossRef] [Green Version]
- Christodoulatos, G.S.; Spyrou, N.; Kadillari, J.; Psallida, S.; Dalamaga, M. The Role of Adipokines in Breast Cancer: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, G.-E.; El Khoury, D. Exercise-Induced Irisin, the Fat Browning Myokine, as a Potential Anticancer Agent. J. Obes. 2019, 2019, 6561726. [Google Scholar] [CrossRef]
- Sumsuzzman, D.M.; Jin, Y.; Choi, J.; Yu, J.-H.; Lee, T.H.; Hong, Y. Pathophysiological Role of Endogenous Irisin against Tumorigenesis and Metastasis: Is It a Potential Biomarker and Therapeutic? Tumour Biol. 2019, 41, 1010428319892790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Tan, X.; Tang, N.; Huang, F.; Chen, Z.; Shi, G. Review of Research on the Role of Irisin in Tumors. OncoTargets Ther. 2020, 13, 4423–4430. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Myint, P.K.; Ito, A.; Appiah, M.G.; Darkwah, S.; Kawamoto, E.; Shimaoka, M. Integrin-Ligand Interactions in Inflammation, Cancer, and Metabolic Disease: Insights Into the Multifaceted Roles of an Emerging Ligand Irisin. Front. Cell Dev. Biol. 2020, 8, 588066. [Google Scholar] [CrossRef]
- Flori, L.; Testai, L.; Calderone, V. The “Irisin System”: From Biological Roles to Pharmacological and Nutraceutical Perspectives. Life Sci. 2021, 267, 118954. [Google Scholar] [CrossRef] [PubMed]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021. [Google Scholar] [CrossRef]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisløff, U.; Tjønna, A.E.; Raastad, T.; et al. Evidence against a Beneficial Effect of Irisin in Humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef] [Green Version]
- Kozak, M. Context Effects and Inefficient Initiation at Non-AUG Codons in Eucaryotic Cell-Free Translation Systems. Mol. Cell. Biol. 1989, 9, 5073–5080. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, E.; Norheim, F.; Thiede, B.; Holen, T.; Ohashi, T.; Schering, L.; Lee, S.; Brenmoehl, J.; Thomas, S.; Drevon, C.A.; et al. Irisin—A Myth Rather than an Exercise-Inducible Myokine. Sci. Rep. 2015, 5, 8889. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, E.; Schering, L.; Buck, F.; Vlach, K.; Schober, H.-C.; Drevon, C.A.; Maak, S. Irisin: Still Chasing Shadows. Mol. Metab. 2020, 34, 124–135. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Villarroya, F. Irisin, Turning up the Heat. Cell Metab. 2012, 15, 277–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akimoto, T.; Pohnert, S.C.; Li, P.; Zhang, M.; Gumbs, C.; Rosenberg, P.B.; Williams, R.S.; Yan, Z. Exercise Stimulates Pgc-1alpha Transcription in Skeletal Muscle through Activation of the P38 MAPK Pathway. J. Biol. Chem. 2005, 280, 19587–19593. [Google Scholar] [CrossRef] [Green Version]
- Spiegelman, B.M. Transcriptional Control of Energy Homeostasis through the PGC1 Coactivators. Novartis Found. Symp. 2007, 286, 3–6, discusssion 6–12, 162–163, 196–203. [Google Scholar]
- Giguère, V.; Yang, N.; Segui, P.; Evans, R.M. Identification of a New Class of Steroid Hormone Receptors. Nature 1988, 331, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Sladek, R.; Carrier, J.; Bader, J.-A.; Richard, D.; Giguère, V. Reduced Fat Mass in Mice Lacking Orphan Nuclear Receptor Estrogen-Related Receptor Alpha. Mol. Cell. Biol. 2003, 23, 7947–7956. [Google Scholar] [CrossRef] [Green Version]
- Laganière, J.; Tremblay, G.B.; Dufour, C.R.; Giroux, S.; Rousseau, F.; Giguère, V. A Polymorphic Autoregulatory Hormone Response Element in the Human Estrogen-Related Receptor Alpha (ERRalpha) Promoter Dictates Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1alpha Control of ERRalpha Expression. J. Biol. Chem. 2004, 279, 18504–18510. [Google Scholar] [CrossRef] [Green Version]
- Mootha, V.K.; Handschin, C.; Arlow, D.; Xie, X.; St Pierre, J.; Sihag, S.; Yang, W.; Altshuler, D.; Puigserver, P.; Patterson, N.; et al. Erralpha and Gabpa/b Specify PGC-1alpha-Dependent Oxidative Phosphorylation Gene Expression That Is Altered in Diabetic Muscle. Proc. Natl. Acad. Sci. USA 2004, 101, 6570–6575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, S.N.; Emter, R.; Hock, M.B.; Knutti, D.; Cardenas, J.; Podvinec, M.; Oakeley, E.J.; Kralli, A. The Estrogen-Related Receptor Alpha (ERRalpha) Functions in PPARgamma Coactivator 1alpha (PGC-1alpha)-Induced Mitochondrial Biogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 6472–6477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-Activated Protein Kinase (AMPK) Action in Skeletal Muscle via Direct Phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.; Li Ji, L. Role of PGC-1α Signaling in Skeletal Muscle Health and Disease. Ann. N. Y. Acad. Sci. 2012, 1271, 110–117. [Google Scholar] [CrossRef]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin Knockout Drives Browning of White Adipose Tissue through Activating the AMPK-PGC1α-Fndc5 Pathway in Muscle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamas, L.; Matafome, P.; Seiça, R. Irisin and Myonectin Regulation in the Insulin Resistant Muscle: Implications to Adipose Tissue: Muscle Crosstalk. J. Diabetes Res. 2015, 2015, 359159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.-Q.; Ding, L.-N.; Zeng, N.-X.; Liu, H.-M.; Zheng, S.-H.; Xu, J.-W.; Li, R.-M. Icariin Induces Irisin/FNDC5 Expression in C2C12 Cells via the AMPK Pathway. Biomed. Pharmacother. 2019, 115, 108930. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin Stimulates Browning of White Adipocytes through Mitogen-Activated Protein Kinase P38 MAP Kinase and ERK MAP Kinase Signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [Green Version]
- Nie, Y.; Liu, D. N-Glycosylation Is Required for FDNC5 Stabilization and Irisin Secretion. Biochem. J. 2017, 474, 3167–3177. [Google Scholar] [CrossRef]
- Moreno, M.; Moreno-Navarrete, J.M.; Serrano, M.; Ortega, F.; Delgado, E.; Sanchez-Ragnarsson, C.; Valdés, S.; Botas, P.; Ricart, W.; Fernández-Real, J.M. Circulating Irisin Levels Are Positively Associated with Metabolic Risk Factors in Sedentary Subjects. PLoS ONE 2015, 10, e0124100. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via AV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Pan, Y.; Li, X.; Cheng, D.; Ju, H.; Tian, J.; Shi, H.; Zhang, Y. Study on the Distribution and Elimination of the New Hormone Irisin in Vivo: New Discoveries Regarding Irisin. Horm. Metab. Res. 2015, 47, 591–595. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Cakmak, T.; Albayrak, S.; Gungor, S.; Colakoglu, N.; Ozercan, I.H. A Comprehensive Immunohistochemical Examination of the Distribution of the Fat-Burning Protein Irisin in Biological Tissues. Peptides 2014, 61, 130–136. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wu, Z.; Lv, Y.; et al. Irisin Reverses Intestinal Epithelial Barrier Dysfunction during Intestinal Injury via Binding to the Integrin AVβ5 Receptor. J. Cell. Mol. Med. 2020, 24, 996–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.; Bi, J.; Yang, L.; Zhang, J.; Wan, Y.; Chen, X.; Wang, Y.; Wu, Z.; Lv, Y.; Wu, R. Serum Irisin Levels Are Decreased in Patients with Sepsis, and Exogenous Irisin Suppresses Ferroptosis in the Liver of Septic Mice. Clin. Transl. Med. 2020, 10, e173. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-S.; Mantzoros, C.S. Regulation of Cell Proliferation and Malignant Potential by Irisin in Endometrial, Colon, Thyroid and Esophageal Cancer Cell Lines. Metabolism 2014, 63, 188–193. [Google Scholar] [CrossRef]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the Exercise-Inducible Myokine Irisin on Malignant and Non-Malignant Breast Epithelial Cell Behavior in Vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef]
- Tekin, S.; Erden, Y.; Sandal, S.; Yilmaz, B. Is Irisin an Anticarcinogenic Peptide? Med. Sci. Int. Med. J. 2015, 4, 2172. [Google Scholar] [CrossRef]
- Shao, L.; Li, H.; Chen, J.; Song, H.; Zhang, Y.; Wu, F.; Wang, W.; Zhang, W.; Wang, F.; Li, H.; et al. Irisin Suppresses the Migration, Proliferation, and Invasion of Lung Cancer Cells via Inhibition of Epithelial-to-Mesenchymal Transition. Biochem. Biophys. Res. Commun. 2017, 485, 598–605. [Google Scholar] [CrossRef]
- Nowinska, K.; Jablonska, K.; Pawelczyk, K.; Piotrowska, A.; Partynska, A.; Gomulkiewicz, A.; Ciesielska, U.; Katnik, E.; Grzegrzolka, J.; Glatzel-Plucinska, N.; et al. Expression of Irisin/FNDC5 in Cancer Cells and Stromal Fibroblasts of Non-Small Cell Lung Cancer. Cancers 2019, 11, 1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, G.-H.; Zhu, T.-Y.; Huang, J. FNDC5 Promotes Paclitaxel Sensitivity of Non-Small Cell Lung Cancers via Inhibiting MDR1. Cell. Signal. 2020, 72, 109665. [Google Scholar] [CrossRef]
- Kong, G.; Jiang, Y.; Sun, X.; Cao, Z.; Zhang, G.; Zhao, Z.; Zhao, Y.; Yu, Q.; Cheng, G. Irisin Reverses the IL-6 Induced Epithelial-Mesenchymal Transition in Osteosarcoma Cell Migration and Invasion through the STAT3/Snail Signaling Pathway. Oncol. Rep. 2017, 38, 2647–2656. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Xu, D.; Chu, K.; Cao, Z.; Sun, X.; Yang, Y. The Effects of MiR-214-3p and Irisin/FNDC5 on the Biological Behavior of Osteosarcoma Cells. Cancer Biother. Radiopharm. 2020, 35, 92–100. [Google Scholar] [CrossRef]
- Shi, G.; Tang, N.; Qiu, J.; Zhang, D.; Huang, F.; Cheng, Y.; Ding, K.; Li, W.; Zhang, P.; Tan, X. Irisin Stimulates Cell Proliferation and Invasion by Targeting the PI3K/AKT Pathway in Human Hepatocellular Carcinoma. Biochem. Biophys. Res. Commun. 2017, 493, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, N.; Huang, Y.; Chen, Y. Irisin Inhibits Pancreatic Cancer Cell Growth via the AMPK-MTOR Pathway. Sci. Rep. 2018, 8, 15247. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Huang, Y.; Liu, Y.; Chen, Y. Irisin Enhances Doxorubicin-Induced Cell Apoptosis in Pancreatic Cancer by Inhibiting the PI3K/AKT/NF-ΚB Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 6085–6096. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, P.; Li, L.; Tang, N.; Huang, F.; Kong, X.; Tan, X.; Shi, G. Irisin Functions to Inhibit Malignant Growth of Human Pancreatic Cancer Cells via Downregulation of the PI3K/AKT Signaling Pathway. OncoTargets Ther. 2019, 12, 7243–7249. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.C.; Leung, P.S. Irisin Is a Positive Regulator for Ferroptosis in Pancreatic Cancer. Mol. Ther. Oncolytics 2020, 18, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Chang, Y.-H.; Lee, H.-H.; Wu, J.-Y.; Huang, J.-X.; Chung, Y.-H.; Hsu, S.-T.; Chow, L.-P.; Wei, K.-C.; Huang, F.-T. Irisin, an Exercise Myokine, Potently Suppresses Tumor Proliferation, Invasion, and Growth in Glioma. FASEB J. 2020, 34, 9678–9693. [Google Scholar] [CrossRef]
- Altay, D.U.; Keha, E.E.; Ozer Yaman, S.; Ince, I.; Alver, A.; Erdogan, B.; Canpolat, S.; Cobanoglu, U.; Mentese, A. Investigation of the Expression of Irisin and Some Cachectic Factors in Mice with Experimentally Induced Gastric Cancer. QJM Int. J. Med. 2016, 109, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Provatopoulou, X.; Georgiou, G.P.; Kalogera, E.; Kalles, V.; Matiatou, M.A.; Papapanagiotou, I.; Sagkriotis, A.; Zografos, G.C.; Gounaris, A. Serum Irisin Levels Are Lower in Patients with Breast Cancer: Association with Disease Diagnosis and Tumor Characteristics. BMC Cancer 2015, 15, 898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-P.; Zhang, X.-F.; Li, H.; Liu, T.-J.; Zhao, Q.-P.; Huang, L.-H.; Cao, Z.-J.; He, L.-M.; Hao, D.-J. Serum Irisin Associates with Breast Cancer to Spinal Metastasis. Medicine (Baltimore) 2018, 97, e0524. [Google Scholar] [CrossRef]
- Gaggini, M.; Cabiati, M.; Del Turco, S.; Navarra, T.; De Simone, P.; Filipponi, F.; Del Ry, S.; Gastaldelli, A.; Basta, G. Increased FNDC5/Irisin Expression in Human Hepatocellular Carcinoma. Peptides 2017, 88, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ke, M.; Ren, Y.; Bi, J.; Du, Z.; Zhang, M.; Wang, Y.; Zhang, L.; Wu, Z.; Lv, Y.; et al. Serum Irisin Predicts Posthepatectomy Complications in Patients with Hepatocellular Carcinoma. Dis. Markers 2019, 2019, 9850191. [Google Scholar] [CrossRef] [Green Version]
- Pazgan-Simon, M.; Zuwała-Jagiełło, J.; Kukla, M.; Grzebyk, E.; Simon, K. Serum Concentrations of Selected Adipokines in Virus-Related Liver Cirrhosis and Hepatocellular Carcinoma. Clin. Exp. Hepatol. 2020, 6, 235–242. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, M.; Zhang, N.; Pan, H.; Lin, G.; Li, N.; Wang, L.; Yang, H.; Yan, K.; Gong, F. Serum and Adipose Tissue MRNA Levels of ATF3 and FNDC5/Irisin in Colorectal Cancer Patients With or Without Obesity. Front. Physiol. 2018, 9, 1125. [Google Scholar] [CrossRef] [PubMed]
- Altay, D.U.; Keha, E.E.; Karagüzel, E.; Menteşe, A.; Yaman, S.O.; Alver, A. The Diagnostic Value of FNDC5/Irisin in Renal Cell Cancer. Int. Braz. J. Urol. Off. J. Braz. Soc. Urol. 2018, 44, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Esawy, M.M.; Abdel-Samd, K.M. The Diagnostic and Prognostic Roles of Serum Irisin in Bladder Cancer. Curr. Probl. Cancer 2020, 44, 100529. [Google Scholar] [CrossRef]
- Aslan, R.; Alp, H.H.; Eryılmaz, R.; Huyut, Z.; Sevim, M.; Araz, Ş.; Ertas, K.; Taken, K. Can the Irisin Be a Biomarker for Prostate Cancer? A Case Control Study. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 505–509. [Google Scholar] [CrossRef]
- Shahidi, S.; Hejazi, J.; Moghimi, M.; Borji, S.; Zabihian, S.; Fathi, M. Circulating Irisin Levels and Redox Status Markers in Patients with Gastric Cancer: A Case-Control Study. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 2847–2851. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, G.; Triantafyllidou, S.; Tarlatzis, B.C.; Papakonstantinou, E. Serum Levels of Irisin and Omentin-1 in Breast Neoplasms and Their Association with Tumor Histology. Int. J. Endocrinol. 2021, 2021, 6656671. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Ozercan, M.R.; Albayrak, S.; Aydin, S.; Bakal, U.; Yilmaz, M.; Kalayci, M.; Yardim, M.; Sarac, M.; et al. Irisin Immunohistochemistry in Gastrointestinal System Cancers. Biotech. Histochem. 2016, 91, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Kuloglu, T.; Celik, O.; Aydin, S.; Hanifi Ozercan, I.; Acet, M.; Aydin, Y.; Artas, G.; Turk, A.; Yardim, M.; Ozan, G.; et al. Irisin Immunostaining Characteristics of Breast and Ovarian Cancer Cells. Cell. Mol. Biol. 2016, 62, 40–44. [Google Scholar]
- Kuloğlu, T.; Artaş, G.; Yardim, M.; Sahin, I.; Aydin, Y.; Beyoğlu, N.; Özercan, I.H.; Yalcin, M.H.; Ugur, K.; Aydin, S. Immunostaining Characteristics of Irisin in Benign and Malignant Renal Cancers. Biotech. Histochem. 2019, 94, 435–441. [Google Scholar] [CrossRef]
- Ugur, K.; Aydin, S.; Kuloglu, T.; Artas, G.; Kocdor, M.A.; Sahin, İ.; Yardim, M.; Ozercan, İ.H. Comparison of Irisin Hormone Expression between Thyroid Cancer Tissues and Oncocytic Variant Cells. Cancer Manag. Res. 2019, 11, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sotelo, D.; Roca-Rivada, A.; Baamonde, I.; Baltar, J.; Castro, A.I.; Domínguez, E.; Collado, M.; Casanueva, F.F.; Pardo, M. Lack of Adipocyte-Fndc5/Irisin Expression and Secretion Reduces Thermogenesis and Enhances Adipogenesis. Sci. Rep. 2017, 7, 16289. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Cancer Cell | Irisin Concentration/Duration | Findings | Reference |
|---|---|---|---|
| KLE, RL95-2 endometrial cancer HT29, MCA38 colon cancer SW579, BHP7 thyroid cancer OE13, OE33 esophageal cancer | 5, 10, 50, 100 nM/36 h | no effect on cell adhesion no effect on colony formation | [76] |
| MDA-MB-231 breast cancer | 0.625–20 nM/24 h | ↓ cell viability ↓ cell migration ↓ NF-κB ↑ caspase 3/7cleavage ↑ apoptosis | [77] |
| LNCaP (androgen receptor positive) prostate cancer DU-145, PC3 (androgen receptor negative) prostate cancer | 0.1, 1, 10 and 100 nM/24 h | ↓ proliferation (cell viability) | [78] |
| A549, NCI-H446 lung cancer | 20 nM/24 h | ↓ proliferation ↓ migration ↓ invasion ↓ EMT ↓ PI3K/AKT ↓ Snail | [79] |
| A549, H1299, H358, H1650 lung cancer | 20 nM/24–96 h | ↓ proliferation ↓ MDR1 ↓ NF-κB | [81] |
| U2OS, MG-63 osteosarcoma | 100 ng/mL/24 h | ↓ proliferation ↓ migration ↓ invasion ↓ EMT ↓ STAT3 ↓ Snail | [82] |
| U2OS osteosarcoma | 25, 50, 100, 200 ng/mL | ↓ proliferation ↓ migration ↓ EMT ↓ invasion | [83] |
| HepG2, SMCC7721 hepatocellular carcinoma | 2.5 nM/24 h | ↑ proliferation ↑ migration ↑ invasion ↑ PI3K | [84] |
| MIA PaCa-2, Panc03.27 pancreatic cancer | 10 and 100 nM/24 h | ↓ proliferation ↓ colony formation ↓ migration ↑ cell cycle arrest (G1) ↓ EMT ↓ vimentin ↑ E-cadherin ↑ AMPK activation ↓ mTOR activation | [85] |
| MIA PaCa-2, BxPC-3, Panc03.27 pancreatic cancer | 5, 10, 50, 100 nM/24 h | ↑ apoptosis ↑ PARP cleavage ↑ caspase-3 cleavage ↓ BCL-2 ↓ BCL-xL ↓ Akt ↓ NF-κB | [86] |
| PANC-1,BxPC-3 pancreatic cancer | 0, 10, 20 and 50 nM/24 h | ↓ proliferation ↑ apoptosis ↓ migration ↓ invasion ↓ Akt | [87] |
| PANC-1 pancreatic cancer | 100 nM/12 h | ↑ erastin-induced apoptosis ↑ ROS levels ↓ GSH levels ↓ NRF2 ↓ P62 | [88] |
| U-87 MG, T98G, LN-18 glioblastoma | 1 μM/72 h | ↓ proliferation ↑ cell cycle arrest ↑ p21 mRNA, protein ↓ invasion ↑ TFPI-2 mRNA, protein | [89] |
| Animal Model | Intervention (Treatment) | Findings | Reference |
|---|---|---|---|
| BALB/c mice | N-nitroso-N-methylurea (MNU) to induce gastric cancer | ↑ FNDC5 mRNA levels in white and brown adipose tissue of mice in pre-cancer and cancer groups ↑ Irisin levels in mice of pre-cancer and cancer groups | [90] |
| Athymic male nude mice injected with human glioblastoma cells (U-87 MG) | irisin 20 μg/day, 14 days | ↓ reduced tumor volume | [89] |
| Participants | Measurements | Findings | Reference |
|---|---|---|---|
| Healthy humans, breast cancer patients (101 invasive ductal) N = 152 | serum irisin levels (ELISA) | ↓ serum irisin levels in breast cancer patients | [91] |
| Breast cancer patients (+ spinal metastasis) N = 148 | serum irisin levels (ELISA) | ↓ serum irisin levels in breast cancer patients with spinal metastasis | [92] |
| Hepatocellular carcinoma patients N = 36 | serum irisin levels (ELISA) | no significant difference in serum irisin levels in HCC patients vs. donors | [93] |
| Patients with hepatocellular carcinoma N = 20 | serum irisin levels (ELISA) | no significant difference in serum irisin levels | [84] |
| Healthy humans, hepatocellular carcinoma patients N = 219 | serum irisin levels (ELISA) | ↓ serum irisin levels in HCC patients ↓ FNDC5/irisin levels in HCC tissues | [94] |
| Healthy humans, hepatocellular carcinoma patients N = 43 | serum irisin levels (ELISA) | ↓ serum irisin levels in HCC patients ↓ FNDC5/irisin levels in HCC tissues | [95] |
| Colorectal cancer patients Obese and non-obese N = 116 | serum irisin levels (ELISA) | ↓ serum irisin levels in CRC patients | [96] |
| Renal cancer patients N = 48 | serum irisin levels (ELISA) | ↑ serum irisin levels in renal cancer patients | [97] |
| Healthy humans, newly diagnosed bladder cancer patients N = 150 | serum irisin levels (ELISA) | ↓ serum irisin levels in bladder cancer patients | [98] |
| Healthy humans, prostate cancer patients N = 80 | serum irisin levels (ELISA) | ↓ serum irisin levels in prostate cancer patients | [99] |
| Healthy humans, gastric cancer patients N = 51 | serum irisin levels (ELISA) | ↑ serum irisin levels in gastric cancer patients | [100] |
| Healthy humans, breast cancer patients N = 213 | serum irisin levels (ELISA) | ↑ serum irisin levels in both benign and malignant breast tumor cases compared to control | [101] |
| Participants | Measurements | Findings | Reference |
|---|---|---|---|
| Healthy humans, tumor tissues (brain, esophagus, stomach liver, pancreas) N = N/A | irisin expression in healthy and cancer tissues (IHC) | ↑ irisin levels in gastrointestinal cancer, grade II astrocytoma | [102] |
| Healthy humans, tumor tissues (breast, cervix, ovaries, endometrium) N = N/A | irisin expression in healthy and cancer tissues (IHC) | ↑ irisin levels in breast, ovarian, cervical and endometrial tumor tissues | [103] |
| Hepatocellular carcinoma patients N = 36 | FNDC5 mRNA levels measured in liver tissues of HCC patients and controls (RT-PCR) | ↑ FNDC5/irisin hepatic mRNA levels | [93] |
| Healthy humans, patients with hepatocellular carcinoma N = 20 | FNDC5 mRNA levels measured in liver tissues of HCC patients and controls (RT-PCR) | ↑ FNDC5 mRNA levels in HCC patients compared to controls | [84] |
| Healthy humans, hepatocellular carcinoma patients N = 219 | FNDC5/irisin expression in HCC tissues | ↓ FNDC5/irisin levels in HCC tissues | [94] |
| Healthy humans, hepatocellular carcinoma patients N = 43 | FNDC5/irisin expression in HCC tissues | ↓ FNDC5/irisin levels in HCC tissues | [95] |
| Colorectal cancer patients obese and non-obese N = 116 | FNDC5/irisin levels in subcutaneous and visceral white adipose tissues (RT-PCR) | no difference between FNDC5 levels in subcutaneous and visceral white adipose tissues. | [96] |
| Renal cancer patients N = 110 | irisin expression in healthy and cancer tissues (IHC) | ↓ FNDC5/irisin in chromophobe renal cell carcinoma | [104] |
| Healthy humans, thyroid cancer patients N = 160 | irisin expression in healthy and cancer tissues (IHC) | ↑ irisin in oncocytic papillary carcinoma ↑ irisin in anaplastic carcinoma | [105] |
| Non-small cell lung cancer patients N = 729 | FNDC5/irisin expression in cancer tissues (IHC and RT-PCR) | ↓ FNDC5/irisin levels in NSCLC tissue ↑ FNDC5/irisin levels in stromal fibroblasts | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiani, E.; Tsakiridis, N.; Kouvelioti, R.; Jaglanian, A.; Klentrou, P. Current Evidence of the Role of the Myokine Irisin in Cancer. Cancers 2021, 13, 2628. https://doi.org/10.3390/cancers13112628
Tsiani E, Tsakiridis N, Kouvelioti R, Jaglanian A, Klentrou P. Current Evidence of the Role of the Myokine Irisin in Cancer. Cancers. 2021; 13(11):2628. https://doi.org/10.3390/cancers13112628
Chicago/Turabian StyleTsiani, Evangelia, Nicole Tsakiridis, Rozalia Kouvelioti, Alina Jaglanian, and Panagiota Klentrou. 2021. "Current Evidence of the Role of the Myokine Irisin in Cancer" Cancers 13, no. 11: 2628. https://doi.org/10.3390/cancers13112628
APA StyleTsiani, E., Tsakiridis, N., Kouvelioti, R., Jaglanian, A., & Klentrou, P. (2021). Current Evidence of the Role of the Myokine Irisin in Cancer. Cancers, 13(11), 2628. https://doi.org/10.3390/cancers13112628