Targeted Liposomal Chemotherapies to Treat Triple-Negative Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cell Lines and Media
2.3. TNBC Patient Tissue Microarray
2.4. Synthesis of Antibody-Liposome-GC/DM1
2.5. Nanoparticle Tracking Analysis
2.6. Transmission Electron Microscopy (TEM)
2.7. In Vitro Drug Cytotoxicity Study
2.8. Western Blotting
2.9. Live-Cell Confocal Microscopy
2.10. Flow Cytometry Analysis
2.11. Immunohistochemistry (IHC) Staining and Scoring
2.12. In Vivo Biodistribution and Ex Vivo Imaging
2.13. Cell Line-Derived Xenograft and In Vivo Treatment
2.14. Patient-Derived Xenograft (PDX) Model and In Vivo Treatment
2.15. Statistical Analysis
3. Results
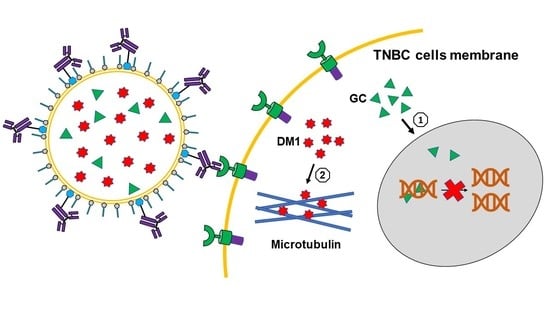
3.1. Construction and Characterization of Targeted Liposomes for Drugs Delivery
3.2. In Vitro Anti-TNBC Cytotoxicity and Synergism Mechanisms
3.3. In Vitro Anti-TNBC Cytotoxicity and Synergism Mechanisms
3.4. Tolerated Dosage in BALB/cJ
3.5. In Vivo Anti-tumor Efficacy in TNBC Cell Line Xenograft Model
3.6. In Vivo Anti-TNBC Efficacy in PDX Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sissung, T.M.; Baum, C.E.; Kirkland, C.T.; Gao, R.; Gardner, E.R.; Figg, W.D. Pharmacogenetics of membrane transporters: An update on current approaches. Mol. Biotechnol. 2009, 44, 152–167. [Google Scholar] [CrossRef]
- Yamada, A.; Ishikawa, T.; Ota, I.; Kimura, M.; Shimizu, D.; Tanabe, M.; Chishima, T.; Sasaki, T.; Ichikawa, Y.; Morita, S.; et al. High expression of ATP-binding cassette transporter ABCC11 in breast tumors is associated with aggressive subtypes and low disease-free survival. Breast Cancer Res. Treat. 2013, 137, 773–782. [Google Scholar] [CrossRef]
- Mahmood, N.A.; Abdulghany, Z.; Al-Sudani, I.M. Expression of Aldehyde Dehydrogenase (ALDH1) and ATP Binding Cassette Transporter G2 (ABCG2) in Iraqi Patients with Colon Cancer and the Relation with Clinicopathological Features. Int. J. Mol. Cell. Med. 2019, 7, 234–240. [Google Scholar]
- Inao, T.; Iida, Y.; Moritani, T.; Okimoto, T.; Tanino, R.; Kotani, H.; Harada, M. Bcl-2 inhibition sensitizes triple-negative human breast cancer cells to doxorubicin. Oncotarget 2018, 9, 25545–25556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.J.; Dhayade, S.; Ferrari, N.; Sims, A.; Johnson, E.; Mason, S.; Dickson, A.; Ryan, K.M.; Kalna, G.; Edwards, J.; et al. MCL-1 is a prognostic indicator and drug target in breast cancer. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer—How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [Green Version]
- Wein, L.; Loi, S. Mechanisms of resistance of chemotherapy in early-stage triple negative breast cancer (TNBC). Breast 2017, 34, S27–S30. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Guo, X.-L. Combinational strategies of metformin and chemotherapy in cancers. Cancer Chemother. Pharmacol. 2016, 78, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, J.; Raki, M.; Bergström, K.A.; Uutela, P.; Lehtinen, K.; Hiltunen, A.; Pikkarainen, J.; Liang, H.; Pitkänen, S.; Määttä, A.-M.; et al. Pre-Targeting and Direct Immunotargeting of Liposomal Drug Carriers to Ovarian Carcinoma. PLoS ONE 2012, 7, e41410. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Huang, L. Nanoparticle delivery of a peptide targeting EGFR signaling. J. Control. Release 2012, 157, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Dehkordi, N.G.; Elahian, F.; Khosravian, P.; Mirzaei, S.A. Intelligent TAT-coupled anti-HER2 immunoliposomes knock downed MDR1 to produce chemosensitize phenotype of multidrug resistant carcinoma. J. Cell. Physiol. 2019, 234, 20769–20778. [Google Scholar] [CrossRef] [PubMed]
- Matusewicz, L.; Filip-Psurska, B.; Psurski, M.; Tabaczar, S.; Podkalicka, J.; Wietrzyk, J.; Ziółkowski, P.; Czogalla, A.; Sikorski, A.F. EGFR-targeted immunoliposomes as a selective delivery system of simvastatin, with potential use in treatment of triple-negative breast cancers. Int. J. Pharm. 2019, 569, 118605. [Google Scholar] [CrossRef]
- Wöll, S.; Dickgiesser, S.; Rasche, N.; Schiller, S.; Scherließ, R. Sortagged anti-EGFR immunoliposomes exhibit increased cytotoxicity on target cells. Eur. J. Pharm. Biopharm. 2019, 136, 203–212. [Google Scholar] [CrossRef]
- Shroff, K.; Kokkoli, E. PEGylated Liposomal Doxorubicin Targeted to α5β1-Expressing MDA-MB-231 Breast Cancer Cells. Langmuir 2012, 28, 4729–4736. [Google Scholar] [CrossRef]
- Jain, A.S.; Goel, P.N.; Shah, S.; Dhawan, V.V.; Nikam, Y.; Gude, R.P.; Nagarsenker, M.S. Tamoxifen guided liposomes for targeting encapsulated anticancer agent to estrogen receptor positive breast cancer cells: In vitro and in vivo evaluation. Biomed. Pharmacother. 2014, 68, 429–438. [Google Scholar] [CrossRef]
- Lu, R.-M.; Chen, M.-S.; Chang, D.-K.; Chiu, C.-Y.; Lin, W.-C.; Yan, S.-L.; Wang, Y.-P.; Kuo, Y.-S.; Yeh, C.-Y.; Lo, A.; et al. Targeted Drug Delivery Systems Mediated by a Novel Peptide in Breast Cancer Therapy and Imaging. PLoS ONE 2013, 8, e66128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosokawa, S.; Tagawa, T.; Niki, H.; Hirakawa, Y.; Nohga, K.; Nagaike, K. Efficacy of immunoliposomes on cancer models in a cell-surface-antigen-density-dependent manner. Br. J. Cancer 2003, 89, 1545–1551. [Google Scholar] [CrossRef] [Green Version]
- Nowsheen, S.; Cooper, T.; Stanley, J.A.; Yang, E.S. Synthetic Lethal Interactions between EGFR and PARP Inhibition in Human Triple Negative Breast Cancer Cells. PLoS ONE 2012, 7, e46614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, T.M.; Iida, M.; Dunn, E.F.; Luthar, N.; Kostopoulos, K.T.; Corrigan, K.L.; Wleklinski, M.J.; Yang, D.; Wisinski, K.B.; Salgia, R.; et al. Nuclear Epidermal Growth Factor Receptor Is a Functional Molecular Target in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2014, 13, 1356–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakaria, Z.; Zulkifle, M.F.; Hasan, W.A.N.W.; Azhari, A.K.; Raub, S.H.A.; Eswaran, J.; Soundararajan, M.; Husain, S.N.A.S. Epidermal growth factor receptor (EGFR) gene alteration and protein overexpression in Malaysian triple-negative breast cancer (TNBC) cohort. OncoTargets Ther. 2019, 12, 7749–7756. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Liu, Z.; Yu, Z. EGFR Promotes the Development of Triple Negative Breast Cancer Through JAK/STAT3 Signaling. Cancer Manag. Res. 2020, 12, 703–717. [Google Scholar] [CrossRef] [Green Version]
- Ali, R.; Wendt, M.K. The paradoxical functions of EGFR during breast cancer progression. Signal Transduct. Target. Ther. 2017, 2, 16042. [Google Scholar] [CrossRef]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, K.; Hung, M.-C.; Yamaguchi, H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res. 2016, 6, 1609–1623. [Google Scholar] [PubMed]
- Bethune, G.; Bethune, E.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar] [PubMed]
- Charakidis, M.; Boyer, M. Targeting MET and EGFR in NSCLC—what can we learn from the recently reported phase III trial of onartuzumab in combination with erlotinib in advanced non-small cell lung cancer? Transl. Lung Cancer Res. 2014, 3, 395–396. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L.; et al. Immunohistochemical and Clinical Characterization of the Basal-Like Subtype of Invasive Breast Carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, R.; Gee, J.; Harper, M. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37, 9–15. [Google Scholar] [CrossRef]
- A Changavi, A.; Shashikala, A.; Ramji, A.S. Epidermal Growth Factor Receptor Expression in Triple Negative and Nontriple Negative Breast Carcinomas. J. Lab. Physicians 2015, 7, 079–083. [Google Scholar] [CrossRef]
- Xu, C.; Li, X.; Liu, P.; Li, M.; Luo, F. Patient-derived xenograft mouse models: A high fidelity tool for individualized medicine (Review). Oncol. Lett. 2018, 17, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, Y.; Kim, S.; Zhang, E.; Tang, Y.; Jaskula-Sztul, R.; Markert, J.M.; Chen, H.; Zhou, L.; Liu, X. (Margaret) Targeted Exosomes for Drug Delivery: Biomanufacturing, Surface Tagging, and Validation. Biotechnol. J. 2020, 15, e1900163. [Google Scholar] [CrossRef]
- Si, Y.; Guan, J.; Xu, Y.; Chen, K.; Kim, S.; Zhou, L.; Jaskula-Sztul, R.; Liu, X.M. Dual-Targeted Extracellular Vesicles to Facilitate Combined Therapies for Neuroendocrine Cancer Treatment. Pharmaceutics 2020, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Kim, S.; Ou, J.; Lu, Y.; Ernst, P.; Chen, K.; Whitt, J.; Carter, A.; Markert, J.M.; Bibb, J.A.; et al. Anti-SSTR2 antibody-drug conjugate for neuroendocrine tumor therapy. Cancer Gene Ther. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Xu, Y.; Guan, J.; Chen, K.; Kim, S.; Yang, E.S.; Zhou, L.; Liu, X.M. Anti-EGFR antibody-drug conjugate for triple-negative breast cancer therapy. Eng. Life Sci. 2021, 21, 37–44. [Google Scholar] [CrossRef]
- Chen, K.; Si, Y.; Ou, J.; Guan, J.-S.; Kim, S.; Ernst, P.; Zhang, Y.; Zhou, L.; Han, X.; Liu, X. Antibody–Drug Conjugate to Treat Meningiomas. Pharmaceutics 2021, 14, 427. [Google Scholar] [CrossRef]
- Huang, S.-T.; Wang, Y.; Chen, Y.-H.; Lin, C.-T.; Li, W.-S.; Wu, H.-C. Liposomal paclitaxel induces fewer hematopoietic and cardiovascular complications than bioequivalent doses of Taxol. Int. J. Oncol. 2018, 53, 1105–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, P.; Nayak, B.; Dey, R. PEGylation in anti-cancer therapy: An overview. Asian J. Pharm. Sci. 2016, 11, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Cui, F.-D.; Choi, M.-K.; Cho, J.-W.; Chung, S.-J.; Shim, C.-K.; Kim, D.-D. Enhanced solubility and stability of PEGylated liposomal paclitaxel: In vitro and in vivo evaluation. Int. J. Pharm. 2007, 338, 317–326. [Google Scholar] [CrossRef]
- Denard, B.; Jiang, S.; Peng, Y.; Ye, J. CREB3L1 as a potential biomarker predicting response of triple negative breast cancer to doxorubicin-based chemotherapy. BMC Cancer 2018, 18, 813. [Google Scholar] [CrossRef] [Green Version]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Bao, X.; Zeng, J.; Huang, H.; Ma, C.; Wang, L.; Wang, F.; Liao, X.; Song, X. Cancer-targeted PEDF-DNA therapy for metastatic colorectal cancer. Int. J. Pharm. 2020, 576, 118999. [Google Scholar] [CrossRef] [PubMed]
- Garbuzenko, O.B.; Kuzmov, A.; Taratula, O.; Pine, S.R.; Minko, T. Strategy to enhance lung cancer treatment by five essential elements: Inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics 2019, 9, 8362–8376. [Google Scholar] [CrossRef]
- Samaddar, S.; Mazur, J.; Boehm, D.; Thompson, D.H. Development And In Vitro Characterization Of Bladder Tumor Cell Targeted Lipid-Coated Polyplex For Dual Delivery Of Plasmids And Small Molecules. Int. J. Nanomed. 2019, 14, 9547–9561. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Liu, Y.; Liu, L.; Huang, Y.; Li, X.; Huang, W. A Multifunction Lipid-Based CRISPR-Cas13a Genetic Circuit Delivery System for Bladder Cancer Gene Therapy. ACS Synth. Biol. 2019, 9, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; Al Faraj, A. siRNA Conjugated Nanoparticles—A Next Generation Strategy to Treat Lung Cancer. Int. J. Mol. Sci. 2019, 20, 6088. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, M.; Yoshida, J. Improvement of Transduction Efficiency of Recombinant Adeno-associated Virus Vector by Entrapment in Multilamellar Liposomes. Jpn. J. Cancer Res. 1998, 89, 352–354. [Google Scholar] [CrossRef]
- Vieweg, J.; Boczkowski, D.; Roberson, K.M.; Edwards, D.W.; Philip, M.; Philip, R.; Rudoll, T.; Smith, C.; Robertson, C.; Gilboa, E. Efficient gene transfer with adeno-associated virus-based plasmids complexed to cationic liposomes for gene therapy of human prostate cancer. Cancer Res. 1995, 55, 2366–2372. [Google Scholar]
- Lins-Austin, B.; Patel, S.; Mietzsch, M.; Brooke, D.; Bennett, A.; Venkatakrishnan, B.; Van Vliet, K.; Smith, A.N.; Long, J.R.; McKenna, R.; et al. Adeno-Associated Virus (AAV) Capsid Stability and Liposome Remodeling During Endo/Lysosomal pH Trafficking. Viruses 2020, 12, 668. [Google Scholar] [CrossRef]
- Tang, X.; Mohuczy, D.; Zhang, Y.C.; Kimura, B.; Galli, S.M.; Phillips, M.I. Intravenous angiotensinogen antisense in AAV-based vector decreases hypertension. Am. J. Physiol. Content 1999, 277, H2392–H2399. [Google Scholar] [CrossRef]
- Elsana, H.; Olusanya, T.O.B.; Carr-Wilkinson, J.; Darby, S.; Faheem, A.; Elkordy, A.A. Evaluation of novel cationic gene based liposomes with cyclodextrin prepared by thin film hydration and microfluidic systems. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjetting, T.; Andresen, T.L.; Christensen, C.L.; Cramer, F.; Poulsen, T.T.; Poulsen, H.S. A simple protocol for preparation of a liposomal vesicle with encapsulated plasmid DNA that mediate high accumulation and reporter gene activity in tumor tissue. Results Pharma Sci. 2011, 1, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Saffari, M.; Moghimi, H.R.; Dass, C.R. Barriers to Liposomal Gene Delivery: From Application Site to the Target. Iran. J. Pharm. Res. IJPR 2016, 15, 3–17. [Google Scholar] [PubMed]
- Zylberberg, C.; Gaskill, K.; Pasley, S.; Matosevic, S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 2017, 24, 441–452. [Google Scholar] [CrossRef]
- Simões, S.; Filipe, A.C.D.S.; Faneca, H.; Mano, M.; Penacho, N.; Düzgünes, N.; de Lima, M.P. Cationic liposomes for gene delivery. Expert Opin. Drug Deliv. 2005, 2, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Suzuki, S.; Kawakami, S.; Yamashita, F.; Hashida, M. The role of dioleoylphosphatidylethanolamine (DOPE) in targeted gene delivery with mannosylated cationic liposomes via intravenous route. J. Control. Release 2005, 108, 484–495. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alton, E.W.F.W.; Armstrong, D.K.; Ashby, D.; Bayfield, K.J.; Bilton, D.; Bloomfield, E.V.; Boyd, A.C.; Brand, J.; Buchan, R.; Calcedo, R.; et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015, 3, 684–691. [Google Scholar] [CrossRef] [Green Version]
- Werner-Wasik, M.; Langer, C.; Movsas, B. Randomized phase II study of amifostine mucosal protection by either subcutaneous injection or rapid IV bolus for patients with inoperable stage II–IIIA/B or stage IV non-small cell lung cancer with oligometastases receiving concurrent radiochemotherapy with carboplatin and paclitaxel followed by optional consolidative chemotherapy: A follow-up study after RTOG 98-01. Semin. Oncol. 2004, 31, 47–51. [Google Scholar] [CrossRef]
- Stopeck, A.T.; Jones, A.; Hersh, E.M.; A Thompson, J.; Finucane, D.M.; Gutheil, J.C.; Gonzalez, R. Phase II study of direct intralesional gene transfer of allovectin-7, an HLA-B7/beta2-microglobulin DNA-liposome complex, in patients with metastatic melanoma. Clin. Cancer Res. 2001, 7, 2285–2291. [Google Scholar] [PubMed]
- Bergen, M.; Chen, R.; Gonzalez, R. Efficacy and safety of HLA-B7/β-2 microglobulin plasmid DNA/lipid complex (Allovectin-7®) in patients with metastatic melanoma. Expert Opin. Biol. Ther. 2003, 3, 377–384. [Google Scholar] [CrossRef]
- Thaker, P.H.; Brady, W.E.; Lankes, H.A.; Odunsi, K.; Bradley, W.H.; Moore, K.N.; Muller, C.Y.; Anwer, K.; Schilder, R.J.; Alvarez, R.D.; et al. A phase I trial of intraperitoneal GEN-1, an IL-12 plasmid formulated with PEG-PEI-cholesterol lipopolymer, administered with pegylated liposomal doxorubicin in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancers: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017, 147, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Atalay, G.; Cardoso, F.; Awada, A.; Piccart, M.J. Novel therapeutic strategies targeting the epidermal growth factor receptor (EGFR) family and its downstream effectors in breast cancer. Ann. Oncol. 2003, 14, 1346–1363. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Opzoomer, J.; Ilieva, K.M.; Gazinska, P.; Hoffmann, R.M.; Mirza, H.; Marlow, R.; Francesch-Domenech, E.; Fittall, M.; Rodriguez, D.D.; et al. Anti-Folate Receptor Alpha–Directed Antibody Therapies Restrict the Growth of Triple-negative Breast Cancer. Clin. Cancer Res. 2018, 24, 5098–5111. [Google Scholar] [CrossRef] [Green Version]
- Perez, E.A. Treatment strategies for advanced hormone receptor-positive and human epidermal growth factor 2-negative breast cancer: The role of treatment order. Drug Resist. Updat. 2016, 24, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Nejad-Ariani, H.; AlThagafi, E.; Kaur, K. Small Peptide Ligands for Targeting EGFR in Triple Negative Breast Cancer Cells. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, S.L.; Lachance, M.P.; Schultz, G.S. Characterization of epidermal growth factor receptor and action on human breast cancer cells in culture. Cancer Res. 1984, 44, 3442–3447. [Google Scholar] [PubMed]
- Flynn, J.F.; Wong, C.; Wu, J.M. Anti-EGFR Therapy: Mechanism and Advances in Clinical Efficacy in Breast Cancer. J. Oncol. 2009, 2009, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; He, H.; Deng, C.; Yin, L.; Zhong, Z. Saporin-loaded CD44 and EGFR dual-targeted nanogels for potent inhibition of metastatic breast cancer in vivo. Int. J. Pharm. 2019, 560, 57–64. [Google Scholar] [CrossRef]
- Garrido, G.; Rabasa, A.; Garrido, C.; Chao, L.; Garrido, F.; Lora, A.M.G.; Sánchez-Ramírez, B. Upregulation of HLA Class I Expression on Tumor Cells by the Anti-EGFR Antibody Nimotuzumab. Front. Pharmacol. 2017, 8, 595. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liao, D.; Chen, C.; Liu, Y.; Chuang, T.-H.; Xiang, R.; Markowitz, D.; Reisfeld, R.A.; Luo, Y. Tumor-Associated Macrophages Regulate Murine Breast Cancer Stem Cells Through a Novel Paracrine EGFR/Stat3/Sox-2 Signaling Pathway. Stem Cells 2012, 31, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Leung, H.W.; Lang, H.-C.; Wang, S.-Y.; Leung, J.H.; Chan, A.L. Cost-utility analysis of stereotactic body radiotherapy plus cetuximab in previously irradiated recurrent squamous cell carcinoma of the head and neck. Expert Rev. Pharmacoeconomics Outcomes Res. 2021, 21, 489–495. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Stöhlmacher-Williams, J.; Davidenko, I.; Licitra, L.; Winquist, E.; Villanueva, C.; Foa, P.; Rottey, S.; Składowski, K.; Tahara, M.; et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): An open-label phase 3 randomised trial. Lancet Oncol. 2013, 14, 697–710. [Google Scholar] [CrossRef] [Green Version]
- Guren, T.K.; Thomsen, M.; Kure, E.H.; Sorbye, H.; Glimelius, B.; Pfeiffer, P.; Österlund, P.; Sigurdsson, F.; Lothe, I.M.B.; Dalsgaard, A.M.; et al. Cetuximab in treatment of metastatic colorectal cancer: Final survival analyses and extended RAS data from the NORDIC-VII study. Br. J. Cancer 2017, 116, 1271–1278. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.-Y.; Park, S.; Kwon, Y. Recent therapeutic trends and promising targets in triple negative breast cancer. Pharmacol. Ther. 2019, 199, 30–57. [Google Scholar] [CrossRef]
- Jonker, D.J.; O’Callaghan, C.J.; Karapetis, C.; Zalcberg, J.R.; Tu, D.; Au, H.-J.; Berry, S.R.; Krahn, M.; Price, T.; Simes, R.J.; et al. Cetuximab for the Treatment of Colorectal Cancer. N. Engl. J. Med. 2007, 357, 2040–2048. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Lenz, H.-J.; Trotta, A.M.; García-Foncillas, J.; Schulten, J.; Audhuy, F.; Merlano, M.; Milano, G. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat. Rev. 2018, 63, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, Y.; Zhang, Y.; Ngo, H.G.; Guan, J.-S.; Chen, K.; Wang, Q.; Singh, A.P.; Xu, Y.; Zhou, L.; Yang, E.S.; et al. Targeted Liposomal Chemotherapies to Treat Triple-Negative Breast Cancer. Cancers 2021, 13, 3749. https://doi.org/10.3390/cancers13153749
Si Y, Zhang Y, Ngo HG, Guan J-S, Chen K, Wang Q, Singh AP, Xu Y, Zhou L, Yang ES, et al. Targeted Liposomal Chemotherapies to Treat Triple-Negative Breast Cancer. Cancers. 2021; 13(15):3749. https://doi.org/10.3390/cancers13153749
Chicago/Turabian StyleSi, Yingnan, Ya Zhang, Hanh Giai Ngo, Jia-Shiung Guan, Kai Chen, Qing Wang, Ajeet Pal Singh, Yuanxin Xu, Lufang Zhou, Eddy S. Yang, and et al. 2021. "Targeted Liposomal Chemotherapies to Treat Triple-Negative Breast Cancer" Cancers 13, no. 15: 3749. https://doi.org/10.3390/cancers13153749
APA StyleSi, Y., Zhang, Y., Ngo, H. G., Guan, J. -S., Chen, K., Wang, Q., Singh, A. P., Xu, Y., Zhou, L., Yang, E. S., & Liu, X. (2021). Targeted Liposomal Chemotherapies to Treat Triple-Negative Breast Cancer. Cancers, 13(15), 3749. https://doi.org/10.3390/cancers13153749