Moderate Altitude Residence Reduces Male Colorectal and Female Breast Cancer Mortality More Than Incidence: Therapeutic Implications?
Abstract
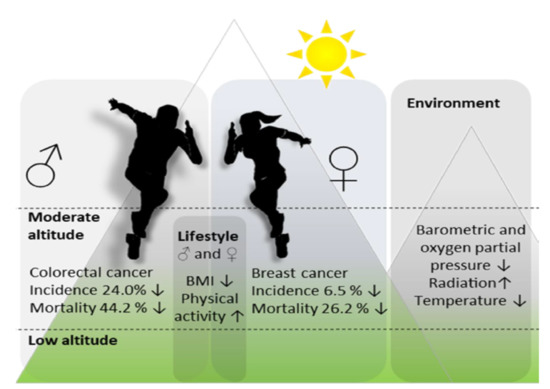
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Incidence and Mortality Data
2.2. Health Interview Survey Results
3. Results
3.1. Incidence and Mortality Data
3.2. Health Interview Survey Results
4. Discussion
4.1. Climate Conditions of Moderate-Altitude Potentially Affecting Cancer Incidence and Mortality Rates
4.2. Non-Climate Factors Potentially Modifying Cancer Incidence and Mortality Rates
4.3. Factors Potentially Explaining Differences between Cancer Incidence and Mortality Rates
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finch, C.E.; Tanzi, R.E. Genetics of aging. Science 1997, 278, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M. Genetic and environmental determinants of early vascular ageing (EVA). Curr. Vasc. Pharmacol. 2012, 10, 700–701. [Google Scholar] [CrossRef]
- Li, Y.; Schoufour, J.; Wang, D.D.; Dhana, K.; Pan, A.; Liu, X.; Song, M.; Liu, G.; Shin, H.J.; Sun, Q.; et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: Prospective cohort study. BMJ 2020, 368, l6669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dato, S.; Rose, G.; Crocco, P.; Monti, D.; Garagnani, P.; Franceschi, C.; Passarino, G. The genetics of human longevity: An intricacy of genes, environment, culture and microbiome. Mech. Ageing Dev. 2017, 165, 147–155. [Google Scholar] [CrossRef]
- Christensen, K.; McGue, M. Genetics: Healthy ageing, the genome and the environment. Nat. Rev. Endocrinol. 2016, 12, 378–380. [Google Scholar] [CrossRef] [Green Version]
- Burtscher, M. Effects of living at higher altitudes on mortality: A narrative review. Aging Dis. 2014, 5, 274–280. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.S.; Otecko, N.O.; Wang, W.; Shi, P.; Wu, D.D.; Zhang, Y.P. Hypoxia potentially promotes Tibetan longevity. Cell Res. 2017, 27, 302–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burtscher, M. Lower mortality rates in those living at moderate altitude. Aging 2016, 8, 2603–2604. [Google Scholar] [CrossRef] [Green Version]
- Faeh, D.; Gutzwiller, F.; Bopp, M.; Group, S.N.C.S. Lower mortality from coronary heart disease and stroke at higher altitudes in Switzerland. Circulation 2009, 120, 495–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.B.; Pan, X.F.; Chen, J.; Cao, A.; Zhang, Y.G.; Xia, L.; Wang, J.; Li, H.; Liu, G.; Pan, A. Combined lifestyle factors, incident cancer, and cancer mortality: A systematic review and meta-analysis of prospective cohort studies. Br. J. Cancer 2020, 122, 1085–1093. [Google Scholar] [CrossRef]
- Pace, M.; Lanzieri, G.; Glickman, M.; Grande, E.; Zupanic, T.; Wojtyniak, B.; Gissler, M.; Cayotte, E.; Agafitei, L. Revision of the European Standard Population; Publications Office of the European Union: Luxembourg, 2013; Available online: https://ec.europa.eu/eurostat/documents/3859598/5926869/KS-RA-13-028-EN.PDF.pdf/e713fa79-1add-44e8-b23d-5e8fa09b3f8f?t=1414782757000 (accessed on 31 August 2021).
- Klimont, J.; Baldaszti, E. Österreichische Gesundheitsbefragung 2014: Hauptergebnisse des Austrian Health Interview Survey (ATHIS) und methodische Dokumentation; Statistics Austria: Wien, Austria, 2015; Available online: https://broschuerenservice.sozialministerium.at/Home/Download?publicationId=542 (accessed on 31 August 2021).
- Thiersch, M.; Swenson, E.R. High Altitude and Cancer Mortality. High Alt. Med. Biol. 2018, 19, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, H.; Zhu, J.; Chen, W.; Wang, L.; Liu, S.; Li, Y.; Liu, Y.; Yin, P.; Liu, J.; et al. Cause-specific mortality for 240 causes in China during 1990-2013: A systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet 2016, 387, 251–272. [Google Scholar] [CrossRef]
- Garrido, D.I.; Garrido, S.M. Cancer risk associated with living at high altitude in Ecuadorian population from 2005 to 2014. Clujul. Med. 2018, 91, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Beall, C.M. Tibetan and Andean patterns of adaptation to high-altitude hypoxia. Hum. Biol. 2000, 72, 201–228. [Google Scholar] [CrossRef] [Green Version]
- Mortimer, E.A., Jr.; Monson, R.R.; MacMahon, B. Reduction in mortality from coronary heart disease in men residing at high altitude. New Engl. J. Med. 1977, 296, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Woolcott, O.O.; Castillo, O.A.; Gutierrez, C.; Elashoff, R.M.; Stefanovski, D.; Bergman, R.N. Inverse association between diabetes and altitude: A cross-sectional study in the adult population of the United States. Obesity 2014, 22, 2080–2090. [Google Scholar] [CrossRef]
- Woolcott, O.O.; Castillo, O.A.; Torres, J.; Damas, L.; Florentini, E. Serum leptin levels in dwellers from high altitude lands. High Alt. Med. Biol. 2002, 3, 245–246. [Google Scholar] [CrossRef]
- Lopez-Pascual, A.; Arévalo, J.; Martínez, J.A.; González-Muniesa, P. Inverse Association Between Metabolic Syndrome and Altitude: A Cross-Sectional Study in an Adult Population of Ecuador. Front Endocrinol. 2018, 9, 658. [Google Scholar] [CrossRef]
- Lopez-Pascual, A.; Bes-Rastrollo, M.; Sayón-Orea, C.; Perez-Cornago, A.; Díaz-Gutiérrez, J.; Pons, J.J.; Martínez-González, M.A.; González-Muniesa, P.; Martínez, J.A. Living at a geographically higher elevation is associated with lower risk of metabolic syndrome: Prospective analysis of the SUN cohort. Front Physiol. 2017, 7, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzati, M.; Horwitz, M.E.; Thomas, D.S.; Friedman, A.B.; Roach, R.; Clark, T.; Murray, C.J.; Honigman, B. Altitude, life expectancy and mortality from ischaemic heart disease, stroke, COPD and cancers: National population-based analysis of US counties. J. Epidemiol. Community Health 2012, 66, e17. [Google Scholar] [CrossRef]
- Lutgens, F.K.; Tarbuck, E.J.; Tusa, D. The Atmosphere; Prentice-Hall: Englewood Cliffs, NJ, USA, 1995; Volume 462. [Google Scholar]
- Blumthaler, M.; Ambach, W.; Ellinger, R. Increase in solar UV radiation with altitude. J. Photochem. Photobiol. B Biol. 1997, 39, 130–134. [Google Scholar] [CrossRef]
- Lo, M.Y.; Daniels, J.D.; Levine, B.D.; Burtscher, M. Sleeping altitude and sudden cardiac death. Am. Heart J. 2013, 166, 71–75. [Google Scholar] [CrossRef]
- Burtscher, M.; Faulhaber, M.; Flatz, M.; Likar, R.; Nachbauer, W. Effects of short-term acclimatization to altitude (3200 m) on aerobic and anaerobic exercise performance. Int. J. Sports Med. 2006, 27, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, H.S.; Uno, M.; Nakamura, H. Suppression of hypoxia-induced HIF-1alpha accumulation by VEGFR inhibitors: Different profiles of AAL993 versus SU5416 and KRN633. Cancer Lett. 2010, 296, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, T.M.; Mäntysaari, M.; Pääkkönen, T.; Jokelainen, J.; Palinkas, L.A.; Hassi, J.; Leppäluoto, J.; Tahvanainen, K.; Rintamäki, H. Autonomic nervous function during whole-body cold exposure before and after cold acclimation. Aviat. Space Environ. Med. 2008, 79, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Van der Lans, A.A.; Hoeks, J.; Brans, B.; Vijgen, G.H.; Visser, M.G.; Vosselman, M.J.; Hansen, J.; Jörgensen, J.A.; Wu, J.; Mottaghy, F.M.; et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 2013, 123, 3395–3403. [Google Scholar] [CrossRef]
- Hidayat, K.; Zhou, H.J.; Shi, B.M. Influence of physical activity at a young age and lifetime physical activity on the risks of 3 obesity-related cancers: Systematic review and meta-analysis of observational studies. Nutr. Rev. 2020, 78, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.B. The health benefits of UV radiation exposure through vitamin D production or non-vitamin D pathways. Blood pressure and cardiovascular disease. Photochem. Photobiol. Sci. 2017, 16, 374–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafi, E.; Khanjani, N.; Ghotbi, M.R.; Masinaei Nejad, M.E. The association of gastrointestinal cancers (esophagus, stomach, and colon) with solar ultraviolet radiation in Iran-an ecological study. Environ. Monit. Assess. 2019, 191, 152. [Google Scholar] [CrossRef]
- Smith, E.S.J.; Schuhmacher, L.; Husson, Z. The naked mole-rat as an animal model in biomedical research: Current perspectives. Open Access Anim. Physiol. 2015, 7, 137–148. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, B.; Ding, M.; Zhou, J.; Yang, C.; Chen, Y. Hypoxia and cancer related pathology. Cancer Lett. 2020, 486, 1–7. [Google Scholar] [CrossRef]
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin. Oncol. Nurs. 2016, 32, 241–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, G.G. Cancer incidence rates and environmental factors: An ecological study. J. Environ. Pathol. Toxicol. Oncol. 2002, 21, 205–212. [Google Scholar] [CrossRef]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Huang, L.; Gao, Y.; Wang, Y.; Chen, S.; Huang, J.; Zheng, W.; Bao, P.; Gong, Y.; Zhang, Y.; et al. Physically active individuals have a 23% lower risk of any colorectal neoplasia and a 27% lower risk of advanced colorectal neoplasia than their non-active counterparts: Systematic review and meta-analysis of observational studies. Br. J. Sports Med. 2020, 54, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chan, A.T.; Slattery, M.L.; Chang-Claude, J.; Potter, J.D.; Gallinger, S.; Caan, B.; Lampe, J.W.; Newcomb, P.A.; Zubair, N.; et al. Influence of Smoking, Body Mass Index, and Other Factors on the Preventive Effect of Nonsteroidal Anti-Inflammatory Drugs on Colorectal Cancer Risk. Cancer Res. 2018, 78, 4790–4799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsong, W.H.; Koh, W.P.; Yuan, J.M.; Wang, R.; Sun, C.L.; Yu, M.C. Cigarettes and alcohol in relation to colorectal cancer: The Singapore Chinese Health Study. Br. J. Cancer 2007, 96, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Smith-Warner, S.A.; Spiegelman, D.; Yaun, S.S.; Adami, H.O.; Beeson, W.L.; van den Brandt, P.A.; Fraser, G.E.; Freudenheim, J.L.; Goldbohm, R.A.; et al. Meat and dairy food consumption and breast cancer: A pooled analysis of cohort studies. Int. J. Epidemiol. 2002, 31, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, D.; Deng, Y.; Liu, K.; Zhou, L.; Li, N.; Zheng, Y.; Hao, Q.; Yang, S.; Wu, Y.; Zhai, Z.; et al. Vitamin D intake, blood vitamin D levels, and the risk of breast cancer: A dose-response meta-analysis of observational studies. Aging 2019, 11, 12708–12732. [Google Scholar] [CrossRef]
- Macacu, A.; Autier, P.; Boniol, M.; Boyle, P. Active and passive smoking and risk of breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2015, 154, 213–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamajima, N.; Hirose, K.; Tajima, K.; Rohan, T.; Calle, E.E.; Heath, C.W.; Coates, R.J.; Liff, J.M.; Talamini, R.; Chantarakul, N.; et al. Alcohol, tobacco and breast cancer-collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br. J. Cancer 2002, 87, 1234–1245. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol. Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Meyerhardt, J.A.; Heseltine, D.; Niedzwiecki, D.; Hollis, D.; Saltz, L.B.; Mayer, R.J.; Thomas, J.; Nelson, H.; Whittom, R.; Hantel, A.; et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J. Clin. Oncol. 2006, 24, 3535–3541. [Google Scholar] [CrossRef]
- Irwin, M.L.; Smith, A.W.; McTiernan, A.; Ballard-Barbash, R.; Cronin, K.; Gilliland, F.D.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The health, eating, activity, and lifestyle study. J. Clin. Oncol. 2008, 26, 3958–3964. [Google Scholar] [CrossRef]
- Jones, L.W.; Viglianti, B.L.; Tashjian, J.A.; Kothadia, S.M.; Keir, S.T.; Freedland, S.J.; Potter, M.Q.; Moon, E.J.; Schroeder, T.; Herndon, J.E.; et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J. Appl. Physiol. 2010, 108, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, J.A.; Wagner, P.D. Exercise-induced arterial hypoxemia. J. Appl. Physiol. 1999, 87, 1997–2006. [Google Scholar] [CrossRef]
- Constantini, K.; Tanner, D.A.; Gavin, T.P.; Harms, C.A.; Stager, J.M.; Chapman, R.F. Prevalence of Exercise-Induced Arterial Hypoxemia in Distance Runners at Sea Level. Med. Sci. Sports Exerc. 2017, 49, 948–954. [Google Scholar] [CrossRef]
- Asano, M.; Kaneoka, K.; Nomura, T.; Asano, K.; Sone, H.; Tsurumaru, K.; Yamashita, K.; Matsuo, K.; Suzuki, H.; Okuda, Y. Increase in serum vascular endothelial growth factor levels during altitude training. Acta Physiol. Scand. 1998, 162, 455–459. [Google Scholar] [CrossRef]
- Mazzone, M.; Dettori, D.; de Oliveira, R.L.; Loges, S.; Schmidt, T.; Jonckx, B.; Tian, Y.M.; Lanahan, A.A.; Pollard, P.; de Almodovar, C.R.; et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 2009, 136, 839–851. [Google Scholar] [CrossRef] [Green Version]
- Vaughan-Shaw, P.G.; Buijs, L.F.; Blackmur, J.P.; Theodoratou, E.; Zgaga, L.; Din, F.V.N.; Farrington, S.M.; Dunlop, M.G. The effect of vitamin D supplementation on survival in patients with colorectal cancer: Systematic review and meta-analysis of randomised controlled trials. Br. J. Cancer 2020, 123, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Callen, D.F.; Li, J.; Zheng, H. Circulating Vitamin D and Overall Survival in Breast Cancer Patients: A Dose-Response Meta-Analysis of Cohort Studies. Integr. Cancer Ther. 2018, 17, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-E.; Paik, H.Y.; Yoon, H.; Lee, J.E.; Kim, N.; Sung, M.-K. Sex- and gender-specific disparities in colorectal cancer risk. World J. Gastroenterol. 2015, 21, 5167–5175. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Ironmonger, L.; Steele, R.J.C.; Ormiston-Smith, N.; Crawford, C.; Seims, A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer 2018, 18, 906. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Colorectal Cancer, Men | Breast Cancer, Women | |||||
|---|---|---|---|---|---|---|
| Altitude (m) | Incidence | SE | 95% CI | Incidence | SE | 95% CI |
| <251 | 88.2 | 1.6 | 85.1;91.3 | 122.8 | 1.6 | 119.7;125.9 |
| 251–500 | 81.4 | 1.0 | 79.4;83.4 | 123.7 | 1.1 | 121.5;125.9 |
| 501–750 | 79.2 | 1.2 | 76.9;81.6 | 126.1 | 1.3 | 123.6;128.6 |
| 751–1000 | 70.9 | 1.9 | 67.2;74.6 | 127.4 | 2.3 | 122.9;131.9 |
| >1000 | 67.0 | 3.1 | 60.9;73.1 | 115.3 | 3.7 | 108.1;122.6 |
| Mortality | SE | 95% CI | Mortality | SE | 95% CI | |
| <251 | 45.0 | 1.2 | 42.7;47.4 | 35.9 | 0.9 | 34.1;37.7 |
| 251–500 | 38.9 | 0.7 | 37.5;40.3 | 32.1 | 0.5 | 31.1;33.1 |
| 501–750 | 33.0 | 0.8 | 31.4;34.6 | 30.7 | 0.7 | 29.3;32.1 |
| 751–1000 | 27.0 | 1.2 | 24.7;29.4 | 27.6 | 1.1 | 25.4;29.8 |
| >1000 | 25.1 | 2.0 | 21.2;29.0 | 26.5 | 1.8 | 23.0;30.0 |
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Altitude, m | <251 | 251–500 | 501–750 | 751–1000 | >1000 | <251 | 251–500 | 501–750 | 751–1000 | >1000 |
| Number | 1361 | 2876 | 1752 | 520 | 204 | 1749 | 3614 | 2171 | 702 | 263 |
| Body mass index, kg/m2 | 27 (4) | 26 (4) | 26 (4) | 26 (4) | 25 (4) * | 25 (5) | 25 (5) | 255 (5) | 25 (5) | 24 (4) * |
| Smokers, daily, % | 17 | 19 | 25 | 24 | 28 * | 25 | 22 | 18 | 19 | 18 * |
| Alcohol consumption on ≥ 2 days/week, % | 20 | 21 | 28 | 29 | 27 * | 11 | 9 | 8 | 5 | 7 * |
| Fruit intake, daily, % | 43 | 46 | 48 | 49 | 51 * | 62 | 66 | 70 | 69 | 64 |
| Vegetable intake, daily, % | 33 | 41 | 42 | 43 | 39 | 47 | 60 | 60 | 61 | 57 * |
| Physical activity, min/week | 195 (316) | 199 (248) | 222 (302) | 232 (294) | 275 (520) * | 163 (222) | 171 (222) | 202 (260) | 207 (239) | 194 (286) * |
| QoL, score (1–5) | 4.1 (0.8) | 4.1 (0.7) | 4.1 (0.7) | 4.2 (0.7) | 4.2 (0.7) | 4.1 (0.8) | 4.1 (0.8) | 4.1 (0.8) | 4.2 (0.7) | 4.1 (0.7) |
| Medical examination | % of men, who never had a colonoscopy | % of women who never had a mammography | ||||||||
| Age >19 years | 63 | 62 | 63 | 60 | 59 | 26 | 27 | 26 | 28 | 33 * |
| Age >50 years | 44 | 41 | 39 | 35 | 37 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burtscher, J.; Millet, G.P.; Renner-Sattler, K.; Klimont, J.; Hackl, M.; Burtscher, M. Moderate Altitude Residence Reduces Male Colorectal and Female Breast Cancer Mortality More Than Incidence: Therapeutic Implications? Cancers 2021, 13, 4420. https://doi.org/10.3390/cancers13174420
Burtscher J, Millet GP, Renner-Sattler K, Klimont J, Hackl M, Burtscher M. Moderate Altitude Residence Reduces Male Colorectal and Female Breast Cancer Mortality More Than Incidence: Therapeutic Implications? Cancers. 2021; 13(17):4420. https://doi.org/10.3390/cancers13174420
Chicago/Turabian StyleBurtscher, Johannes, Grégoire P. Millet, Kathrin Renner-Sattler, Jeannette Klimont, Monika Hackl, and Martin Burtscher. 2021. "Moderate Altitude Residence Reduces Male Colorectal and Female Breast Cancer Mortality More Than Incidence: Therapeutic Implications?" Cancers 13, no. 17: 4420. https://doi.org/10.3390/cancers13174420
APA StyleBurtscher, J., Millet, G. P., Renner-Sattler, K., Klimont, J., Hackl, M., & Burtscher, M. (2021). Moderate Altitude Residence Reduces Male Colorectal and Female Breast Cancer Mortality More Than Incidence: Therapeutic Implications? Cancers, 13(17), 4420. https://doi.org/10.3390/cancers13174420