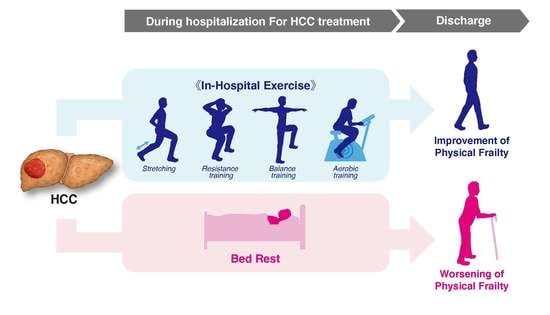
Effects of In-Hospital Exercise on Frailty in Patients with Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Differences in Patient Characteristics between the Exercise and Non-Exercise Groups
2.2. Differences in LFI Changes during Hospitalization between the Exercise and Non-Exercise Groups
2.3. Logistic Regression Analysis and Decision-Tree Analysis for Improvement of LFI
2.4. Differences in Patient Characteristics between the Exercise and Non-Exercise Groups after Propensity Score Matching
2.5. Differences in LFI Changes during Hospitalization between the Exercise and Non-Exercise Groups after Propensity Score Matching
2.6. Logistic Regression Analysis and Decision-Tree Analysis for Improvement of LFI after Propensity Score Matching
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Subjects
4.3. Exercise Regimen
4.3.1. Stretching
4.3.2. Resistance Training
4.3.3. Balance Training
4.3.4. Aerobic Training
4.4. Nutrition and Diet Therapy
4.5. Branched-Chain Amino Acid Supplementation
4.6. Measurement of Liver Frailty Index
4.7. Changes in Liver Frailty Index
4.8. Measurement of Skeletal Muscle Index, and Visceral Fat Area
4.9. Diagnosis of Sarcopenia
4.10. Diagnosis, Tumor Node Metastasis Staging, and Treatment of Hepatocellular Carcinoma
4.11. Biochemical Tests
4.12. Propensity Score Matching
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorenzo-Lopez, L.; Maseda, A.; de Labra, C.; Regueiro-Folgueira, L.; Rodriguez-Villamil, J.L.; Millan-Calenti, J.C. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. 2017, 17, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walston, J.; Buta, B.; Xue, Q.L. Frailty Screening and Interventions: Considerations for Clinical Practice. Clin. Geriatr. Med. 2018, 34, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Ethun, C.G.; Bilen, M.A.; Jani, A.B.; Maithel, S.K.; Ogan, K.; Master, V.A. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J. Clin. 2017, 67, 362–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirota, K.; Kawaguchi, T.; Koya, S.; Nagamatsu, A.; Tomita, M.; Hashida, R.; Nakano, D.; Niizeki, T.; Matsuse, H.; Shiba, N.; et al. Clinical utility of the Liver Frailty Index for predicting muscle atrophy in chronic liver disease patients with hepatocellular carcinoma. Hepatol. Res. 2020, 50, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Yoh, K.; Enomoto, H.; Ikeda, N.; Aizawa, N.; Koriyama, T.; Nishimura, T.; Nishiguchi, S.; Iijima, H. Anthropometric Measurements and Frailty in Patients with Liver Diseases. Diagnostics 2020, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Yoh, K.; Enomoto, H.; Iwata, Y.; Sakai, Y.; Kishino, K.; Shimono, Y.; Ikeda, N.; Takashima, T.; Aizawa, N.; et al. Health-Related Quality of Life and Frailty in Chronic Liver Diseases. Life 2020, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, S., Jr.; Khromava, M.; Schiano, T.D.; Lin, H.M.; Kim, S. Standardized measures of frailty predict hospital length of stay following orthotopic liver transplantation for hepatocellular carcinoma. Clin. Transplant. 2019, 33, e13746. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Shimada, M.; Morine, Y.; Imura, S.; Ikemoto, T.; Arakawa, Y.; Saito, Y.; Yoshikawa, M.; Miyazaki, K. Significance of Frailty in Prognosis After Hepatectomy for Elderly Patients with Hepatocellular Carcinoma. Ann. Surg. Oncol. 2020, 28, 439–446. [Google Scholar] [CrossRef]
- Lai, J.C.; Covinsky, K.E.; Dodge, J.L.; Boscardin, W.J.; Segev, D.L.; Roberts, J.P.; Feng, S. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017, 66, 564–574. [Google Scholar] [CrossRef]
- Lai, J.C.; Rahimi, R.S.; Verna, E.C.; Kappus, M.R.; Dunn, M.A.; McAdams-DeMarco, M.; Haugen, C.E.; Volk, M.L.; Duarte-Rojo, A.; Ganger, D.R.; et al. Frailty Associated With Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology 2019, 156, 1675–1682. [Google Scholar] [CrossRef]
- Hirota, K.; Kawaguchi, T.; Hashida, R.; Koya, S.; Bekki, M.; Goshima, N.; Yoshiyama, T.; Otsuka, T.; Nozoe, R.; Nakano, D.; et al. Profiles Associated with Sarcopenia in Hepatoma Patients Underwent Transcatheter Arterial Chemoembolization: A Data-Mining Analysis. J. Cahexia Sarcopenia Muscle Clin. Rep. 2018, 3, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Williams, F.R.; Berzigotti, A.; Lord, J.M.; Lai, J.C.; Armstrong, M.J. Review article: Impact of exercise on physical frailty in patients with chronic liver disease. Aliment. Pharmacol. Ther. 2019, 50, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H. Importance of rehabilitation in cancer treatment and palliative medicine. Jpn. J. Clin. Oncol. 2011, 41, 733–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koya, S.; Kawaguchi, T.; Hashida, R.; Goto, E.; Matsuse, H.; Saito, H.; Hirota, K.; Taira, R.; Matsushita, Y.; Imanaga, M.; et al. Effects of in-hospital exercise on liver function, physical ability, and muscle mass during treatment of hepatoma in patients with chronic liver disease. Hepatol. Res. 2017, 47, E22–E34. [Google Scholar] [CrossRef] [Green Version]
- Koya, S.; Kawaguchi, T.; Hashida, R.; Hirota, K.; Bekki, M.; Goto, E.; Yamada, M.; Sugimoto, M.; Hayashi, S.; Goshima, N.; et al. Effects of in-hospital exercise on sarcopenia in hepatoma patients who underwent transcatheter arterial chemoembolization. J. Gastroenterol. Hepatol. 2019, 34, 580–588. [Google Scholar] [CrossRef]
- Oldervoll, L.M.; Loge, J.H.; Lydersen, S.; Paltiel, H.; Asp, M.B.; Nygaard, U.V.; Oredalen, E.; Frantzen, T.L.; Lesteberg, I.; Amundsen, L.; et al. Physical exercise for cancer patients with advanced disease: A randomized controlled trial. Oncologist 2011, 16, 1649–1657. [Google Scholar] [CrossRef] [Green Version]
- Marechal, R.; Fontvieille, A.; Parent-Roberge, H.; Fulop, T.; Riesco, E.; Pavic, M.; Dionne, I.J. Effect of a mixed-exercise program on physical capacity and sedentary behavior in older adults during cancer treatments. Aging Clin. Exp. Res. 2019, 31, 1583–1589. [Google Scholar] [CrossRef]
- Zimmer, P.; Trebing, S.; Timmers-Trebing, U.; Schenk, A.; Paust, R.; Bloch, W.; Rudolph, R.; Streckmann, F.; Baumann, F.T. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: A randomized controlled trial. Support. Care Cancer 2018, 26, 615–624. [Google Scholar] [CrossRef]
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvao, D.A.; Chinapaw, M.J.; et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.D.; Lee, P.H.; Hsiao, D.J.; Huang, S.W.; Tsauo, J.Y.; Chen, H.C.; Liou, T.H. Effects of Protein Supplementation Combined with Exercise Intervention on Frailty Indices, Body Composition, and Physical Function in Frail Older Adults. Nutrients 2018, 10, 1916. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Smith, L.; Hamer, M. Gender-specific risk factors for incident sarcopenia: 8-year follow-up of the English longitudinal study of ageing. J. Epidemiol. Community Health 2019, 73, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Huenchullan, S.F.; Tam, C.S.; Ban, L.A.; Ehrenfeld-Slater, P.; McLennan, S.V.; Twigg, S.M. Skeletal muscle adiponectin induction in obesity and exercise. Metabolism 2020, 102, 154008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manieri, E.; Herrera-Melle, L.; Mora, A.; Tomas-Loba, A.; Leiva-Vega, L.; Fernandez, D.I.; Rodriguez, E.; Moran, L.; Hernandez-Cosido, L.; Torres, J.L.; et al. Adiponectin accounts for gender differences in hepatocellular carcinoma incidence. J. Exp. Med. 2019, 216, 1108–1119. [Google Scholar] [CrossRef]
- Sanz, B.; Arrieta, H.; Hervas, G.; Rezola-Pardo, C.; Ruiz-Litago, F.; Iturburu, M.; Gil, S.M.; Rodriguez-Larrad, A.; Irazusta, J. Serum adiponectin is associated with body composition and cognitive and psychological status in older adults living in long-term nursing homes. Exp. Gerontol. 2019, 121, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Nishio, N.; Tanaka, T.; Ohji, S.; Otobe, Y.; Koyama, S.; Sato, A.; Suzuki, M.; et al. Plasma Amino Acid Concentrations Are Associated with Muscle Function in Older Japanese Women. J. Nutr. Health Aging 2018, 22, 819–823. [Google Scholar] [CrossRef]
- Uojima, H.; Sakurai, S.; Hidaka, H.; Kinbara, T.; Sung, J.H.; Ichita, C.; Tokoro, S.; Masuda, S.; Sasaki, A.; Koizumi, K.; et al. Effect of branched-chain amino acid supplements on muscle strength and muscle mass in patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1402–1407. [Google Scholar] [CrossRef]
- Borack, M.S.; Volpi, E. Efficacy and Safety of Leucine Supplementation in the Elderly. J. Nutr. 2016, 146, 2625S–2629S. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, F.J.; Matias, C.N.; Monteiro, C.P.; Valamatos, M.J.; Reis, J.F.; Tavares, F.; Batista, A.; Domingos, C.; Alves, F.; Sardinha, L.B.; et al. Leucine Metabolites Do Not Enhance Training-induced Performance or Muscle Thickness. Med. Sci. Sports Exerc. 2019, 51, 56–64. [Google Scholar] [CrossRef]
- Leahy, D.T.; Pintauro, S.J. Branched-chain amino Acid plus glucose supplement reduces exercise-induced delayed onset muscle soreness in college-age females. ISRN Nutr. 2013, 2013, 921972. [Google Scholar] [CrossRef] [Green Version]
- Weissenborn, K. Hepatic Encephalopathy: Definition, Clinical Grading and Diagnostic Principles. Drugs 2019, 79, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R. Consensus on Exercise Reporting Template (CERT): Explanation and Elaboration Statement. Br. J. Sports Med. 2016, 50, 1428–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, M.D.; Lukasik, L.; Muth, T.; Esposito, P.; Haapala, H.; Gordon, P.M.; IglayReger, H.; Hurvitz, E.A. Recumbent cross-training is a feasible and safe mode of physical activity for significantly motor-impaired adults with cerebral palsy. Arch. Phys. Med. Rehabil. 2013, 94, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Endo, R.; Kohgo, Y.; Ohtake, T.; Ueno, Y.; Kato, A.; Suzuki, K.; Shiraki, R.; Moriwaki, H.; Habu, D.; et al. Guidelines on nutritional management in Japanese patients with liver cirrhosis from the perspective of preventing hepatocellular carcinoma. Hepatol. Res. 2012, 42, 621–626. [Google Scholar] [CrossRef]
- Fukui, H.; Saito, H.; Ueno, Y.; Uto, H.; Obara, K.; Sakaida, I.; Shibuya, A.; Seike, M.; Nagoshi, S.; Segawa, M.; et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J. Gastroenterol. 2016, 51, 629–650. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, T.; Shiraishi, K.; Ito, T.; Suzuki, K.; Koreeda, C.; Ohtake, T.; Iwasa, M.; Tokumoto, Y.; Endo, R.; Kawamura, N.H.; et al. Branched-chain amino acids prevent hepatocarcinogenesis and prolong survival of patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2014, 12, 1012–1018.e1. [Google Scholar] [CrossRef]
- Lai, J.C.; Covinsky, K.E.; McCulloch, C.E.; Feng, S. The Liver Frailty Index Improves Mortality Prediction of the Subjective Clinician Assessment in Patients With Cirrhosis. Am. J. Gastroenterol. 2018, 113, 235–242. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Mazzuca, F.; Onesti, C.E.; Roberto, M.; Di Girolamo, M.; Botticelli, A.; Begini, P.; Strigari, L.; Marchetti, P.; Muscaritoli, M. Lean body mass wasting and toxicity in early breast cancer patients receiving anthracyclines. Oncotarget 2018, 9, 25714–25722. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Kudo, M.; Chung, H.; Osaki, Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): Its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J. Gastroenterol. 2003, 38, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Arii, S.; Sata, M.; Sakamoto, M.; Shimada, M.; Kumada, T.; Shiina, S.; Yamashita, T.; Kokudo, N.; Tanaka, M.; Takayama, T.; et al. Management of hepatocellular carcinoma: Report of Consensus Meeting in the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol. Res. 2010, 40, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Hashida, R.; Kawaguchi, T.; Koya, S.; Hirota, K.; Goshima, N.; Yoshiyama, T.; Otsuka, T.; Bekki, M.; Iwanaga, S.; Nakano, D.; et al. Impact of cancer rehabilitation on the prognosis of patients with hepatocellular carcinoma. Oncol. Lett. 2020, 19, 2355–2367. [Google Scholar] [CrossRef] [PubMed]
- Shimose, S.; Tanaka, M.; Iwamoto, H.; Niizeki, T.; Shirono, T.; Aino, H.; Noda, Y.; Kamachi, N.; Okamura, S.; Nakano, M.; et al. Prognostic impact of transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation in patients with unresectable hepatocellular carcinoma: Comparison with TACE alone using decision-tree analysis after propensity score matching. Hepatol. Res. 2019, 49, 919–928. [Google Scholar] [CrossRef]
- Shigeto, K.; Kawaguchi, T.; Koya, S.; Hirota, K.; Tanaka, T.; Nagasu, S.; Fukahori, M.; Ushijima, T.; Matsuse, H.; Miwa, K.; et al. Profiles Combining Muscle Atrophy and Neutrophil-to-Lymphocyte Ratio Are Associated with Prognosis of Patients with Stage IV Gastric Cancer. Nutrients 2020, 12, 1884. [Google Scholar] [CrossRef] [PubMed]




| Variable | Reference Value | Exercise | Non-Exercise | |||
|---|---|---|---|---|---|---|
| Median (IQR) | Range (Min–Max) | Median (IQR) | Range (Min–Max) | p-Value | ||
| Number | N/A | 114 | N/A | 67 | N/A | N/A |
| Age (years) | N/A | 79 (74–83) | 57–94 | 76 (70–81) | 49–92 | 0.0200 |
| Sex (female/male) | N/A | 42.1%/57.9%(48/66) | N/A | 38.8%/61.2%(26/41) | N/A | 0.6629 |
| Body mass index (kg/m2) | 18.5–24.9 | 22.9 (21.0–25.2) | 15.6–32.7 | 23.6 (21.5–25.8) | 18.1–31.3 | 0.2118 |
| Etiology of liver disease (Alcohol/HBV/HCV/NAFLD/Others) | N/A | 7.9%/11.4%/57.0%/14.0%/9.7% (9/13/65/16/11) | N/A | 16.4%/7.5%/58.2%/4.5%/13.4% (11/5/39/3/9) | N/A | 0.1048 |
| Treatment of HCC (HAIC/TACE/Sorafenib/Ramucirumab/Lenvatinib/Others) | N/A | 3.5%/85.1%/1.8%/0.9%/7.9%/0.9% (4/97/2/1/9/1) | N/A | 0.0%/98.5%/0.0%/0.0%/1.5%/0.0% (0/66/0/0/1/0) | N/A | 0.1227 |
| TNM stage (I/II/III/IVa/IVb) | N/A | 16.7%/37.7%/35.1%/6.1%/4.4% (19/43/40/7/5) | N/A | 13.4%/38.8%/31.3%/9.0%/7.5% (9/26/21/6/5) | N/A | 0.7975 |
| Albumin-bilirubin (ALBI) grade (1/2/3) | N/A | 67.5%/28.1%/4.4% (77/32/5) | N/A | 65.7%/32.8%/1.5% (44/22/1) | N/A | 0.4943 |
| BCAA supplementation (yes/no) | N/A | 22.8%/77.2% (26/88) | N/A | 35.8%/64.2% (24/43) | N/A | 0.0587 |
| LFI (score) | <3.20 | 3.84 (3.27–4.27) | 2.37–5.48 | 3.57 (3.08–4.03) | 1.95–5.51 | 0.0234 |
| LFI (Robust/Pre-frail-Frail) | N/A | 20.2%/79.8% (23/91) | N/A | 28.4%/71.6% (19/48) | N/A | 0.3368 |
| Sarcopenia (Sarcopenia/Non-sarcopenia) | N/A | 32.5%/67.5% (37/77) | N/A | 22.4%/77.6% (15/52) | N/A | 0.1484 |
| Barthel Index (0–100) | N/A | 100 (100–100) | 50–100 | 100 (100–100) | 55–100 | 0.0342 |
| SMI (low/high) | N/A | 49.1%/50.9% (56/58) | N/A | 62.7%/37.3% (42/25) | N/A | 0.0770 |
| VFA (cm2) | N/A | 86.9 (54.5–152.3) | 9.7–310.9 | 100.4 (72.4–120.0) | 17.6–206.5 | 0.6327 |
| Hospitalization (days) | N/A | 9 (9–11) | 6–24 | 12 (11–13) | 6–22 | 0.0001 |
| Exercise days | N/A | 4 (3–5) | 1–13 | N/A | N/A | N/A |
| Exercise implementation rate (%) | N/A | 100.0 (75.0–100.0) | 25.0–100.0 | N/A | N/A | N/A |
| Biochemical examinations | ||||||
| alpha-fetoprotein (AFP) (ng/mL) | ≤10.0 | 10.0 (3.6–85.4) | 1.0–64458.0 | 11.7 (3.3–275.3) | 1.0–146556.0 | 0.8543 |
| des-γ-carboxy prothrombin (DCP) (mAU/mL) | ≤40.0 | 49.0 (22.0–239.0) | 6.3–292689.0 | 110.5 (24.8–960.5) | 1.1–138123.0 | 0.0600 |
| AST (IU/L) | 13–30 | 32 (25–43) | 4–111 | 33 (26–47) | 16–152 | 0.4198 |
| ALT (IU/L) | 10–30 | 23 (18–33) | 5–126 | 23 (14–33) | 6–139 | 0.4038 |
| Lactate dehydrogenase (IU/L) | 120–240 | 205 (183–234) | 114–428 | 204 (189–229) | 153–487 | 0.7743 |
| ALP (IU/L) | 115–359 | 317 (239–413) | 150–3021 | 372 (252–495) | 148–967 | 0.2476 |
| GGT (IU/L) | 13–64 | 47 (29–85) | 12–659 | 53 (35–90) | 10–421 | 0.5293 |
| Cholinesterase (U/L) | 201–421 | 208 (172–238) | 73–411 | 214 (133–259) | 63–380 | 0.9194 |
| Total protein (g/dL) | 6.6–8.1 | 7.1 (6.8–7.4) | 5.9–8.7 | 7.2 (6.7–7.6) | 5.9–8.2 | 0.3803 |
| Albumin (g/dL) | 4.1–5.1 | 3.8 (3.3–4.1) | 2.0–4.7 | 3.8 (3.3–4.2) | 2.6–4.6 | 0.7932 |
| Total bilirubin (mg/dL) | 0.20–1.20 | 0.70 (0.50–1.00) | 0.30–2.90 | 0.80 (0.60–1.10) | 0.30–3.20 | 0.2772 |
| BUN (mg/dL) | 8.0–22.0 | 18.2 (13.2–22.8) | 7.0–59.0 | 17.0 (12.0–21.0) | 10.0–53.0 | 0.2450 |
| Creatinine (mg/dL) | 0.65–1.07 | 0.82 (0.67–1.00) | 0.44–5.11 | 0.79 (0.64–0.92) | 0.41–3.80 | 0.3941 |
| eGFR (mL/min/1.73 m2) | >90.0 | 63.5 (49.6–74.3) | 7.0–140.9 | 66.5 (54.2–81.7) | 9.5–116.3 | 0.0726 |
| Sodium (mmol/L) | 138–145 | 140 (139–142) | 127–146 | 140 (139–142) | 130–147 | 0.5804 |
| Total cholesterol (mg/dL) | 150–199 | 167 (144–193) | 89–235 | 170 (145–198) | 104–267 | 0.6482 |
| Triglyceride (mg/dL) | 35–149 | 93 (73–126) | 29–305 | 97 (76–133) | 27–301 | 0.2524 |
| Creatine kinase (U/L) | 59–248 | 88 (60–129) | 17–656 | 81 (58–124) | 31–338 | 0.5646 |
| Glucose (mg/dL) | 0–99 | 113 (97–135) | 65–495 | 111 (99–152) | 82–316 | 0.6741 |
| HbA1c (%) | 4.3–5.8 | 6.1 (5.7–6.6) | 5.1–10.5 | 6.1 (5.7–6.8) | 4.7–9.3 | 0.9942 |
| Ammonia (µg/dL) | 13–86 | 46 (35–67) | 17–180 | 41 (31–70) | 13–130 | 0.2780 |
| Prothrombin activity (%) | 80–120 | 93 (84–103) | 30–130 | 96 (79–108) | 11–130 | 0.8135 |
| Hemoglobin (g/dL) | 13.7–16.8 | 12.5 (10.8–13.6) | 7.3–15.7 | 12.6 (10.5–13.9) | 8.1–16.2 | 0.8994 |
| White blood cell count (/µL) | 3300–8600 | 4400 (3500–5450) | 1000–8900 | 4600 (3300–5900) | 500–8700 | 0.9104 |
| Platelet count (× 103/mm3) | 15.8–34.8 | 134.0 (93.0–179.5) | 62.0–368.0 | 129.0 (80.0–180.0) | 40.0–419.0 | 0.4327 |
| Neutrophil/Lymphocyte Ratio | 0.86–2.77 | 2.46 (1.71–3.67) | 0.62–11.17 | 2.44 (1.68–3.45) | 0.50–11.71 | 0.5402 |
| Variable | Reference Value | Exercise | Non-Exercise | |||
|---|---|---|---|---|---|---|
| Median (IQR) | Range (Min–Max) | Median (IQR) | Range (Min–Max) | p-Value | ||
| Number | N/A | 59 | N/A | 59 | N/A | N/A |
| Age (years) | N/A | 77 (73–81) | 59–94 | 77 (72–81) | 58–92 | 0.9227 |
| Sex (female/male) | N/A | 33.9%/66.1% (20/39) | N/A | 39.0%/61.0% (23/36) | N/A | 0.5661 |
| Body mass index (kg/m2) | 18.5–24.9 | 23.2 (21.0–25.9) | 17.1–31.3 | 23.5 (21.4–25.1) | 18.1–31.3 | 0.8866 |
| Etiology of liver disease (Alcohol/HBV/HCV/NAFLD/Others) | N/A | 6.8%/15.2%/50.9%/15.2%/11.9% (4/9/30/9/7) | N/A | 18.6%/6.8%/55.9%/5.1%/13.6% (11/4//33/3/8) | N/A | 0.0780 |
| Treatment of HCC (HAIC/TACE/Sorafenib/Lenvatinib/Other) | N/A | 1.7%/89.8%/1.7%/5.1%/1.7% (1/53/1/3/1) | N/A | 0.0%/98.3%/0.0%/1.7%/0.0% (0/58/0/1/0) | N/A | 0.3764 |
| TNM stage (I/II/III/IVa/IVb) | N/A | 15.2%/39.0%/33.9%/5.1%/6.8% (9/23/20/3/4) | N/A | 10.1%/44.1%/33.9%/6.8%/5.1% (6/26/20/4/3) | N/A | 0.8991 |
| ALBI grade (1/2/3) | N/A | 64.4%/30.5%/5.1% (38/18/3) | N/A | 67.0%/28.8%/1.7% (41/17/1) | N/A | 0.5648 |
| BCAA supplementation (yes/no) | N/A | 23.7%/76.3% (14/45) | N/A | 32.2%/67.8% (19/40) | N/A | 0.3051 |
| LFI | <3.20 | 3.80 (3.22–4.30) | 2.37–4.87 | 3.51 (3.08–4.10) | 2.21–5.51 | 0.1230 |
| Frailty determination by LFI (Robust/Pre-frail-Frail) | N/A | 22.0%/78.0% (13/46) | N/A | 28.8%/71.2% (17/42) | N/A | 0.5382 |
| Sarcopenia (Sarcopenia/Non-sarcopenia) | N/A | 22.0%/78.0% (13/46) | N/A | 25.4%/74.6% (15/44) | N/A | 0.6652 |
| Barthel Index (0–100) | N/A | 100 (100–100) | 65–100 | 100 (100–100) | 55–100 | 0.2043 |
| SMI (low/high) | N/A | 49.2%/50.9% (29/30) | N/A | 67.8%/32.2% (40/19) | N/A | 0.0399 |
| VFA (cm2) | N/A | 96.1 (66.2–150.0) | 10.1–252.0 | 98.3 (61.4–122.6) | 17.6–206.5 | 0.5379 |
| Hospitalization (days) | N/A | 9 (9–11) | 6–24 | 12 (11–13) | 6–20 | 0.0001 |
| Exercise days | N/A | 4 (3–5) | 1–13 | N/A | N/A | N/A |
| Exercise implementation rate (%) | N/A | 83.0 (75.0–100.0) | 25.0–100.0 | N/A | N/A | N/A |
| Biochemical examinations | ||||||
| AFP (ng/mL) | ≤10.0 | 11.3 (4.1–147.0) | 1.0–64458.0 | 12.7 (2.8–267.0) | 1.0–146556.0 | 0.5611 |
| DCP (mAU/mL) | ≤40.0 | 54.0 (22.0–450.0) | 6.3–292689.0 | 107.0 (25.0–841.0) | 1.1–86289.0 | 0.2876 |
| AST (IU/L) | 13–30 | 35 (26–44) | 14–103 | 34 (26–47) | 16–152 | 0.7284 |
| ALT (IU/L) | 10–30 | 28 (18–36) | 5–126 | 23 (14–33) | 8–139 | 0.1171 |
| Lactate dehydrogenase (IU/L) | 120–240 | 200 (182–243) | 143–428 | 205 (189–229) | 153–487 | 0.8230 |
| ALP (IU/L) | 115–359 | 316 (229–424) | 150–3021 | 363 (239–447) | 148–967 | 0.4625 |
| GGT (IU/L) | 13–64 | 52 (31–112) | 13–659 | 53 (35–91) | 10–421 | 0.6926 |
| Cholinesterase (U/L) | 201–421 | 218 (147–239) | 82–411 | 214 (145–261) | 63–380 | 0.9036 |
| Total protein (g/dL) | 6.6–8.1 | 7.1 (6.8–7.4) | 5.9–8.7 | 7.2 (6.8–7.6) | 5.9–8.2 | 0.2458 |
| Albumin (g/dL) | 4.1–5.1 | 3.8 (3.3–4.1) | 2.0–4.4 | 3.9 (3.3–4.2) | 2.6–4.6 | 0.5206 |
| Total bilirubin (mg/dL) | 0.20–1.20 | 0.80 (0.50–1.00) | 0.30–2.90 | 0.80 (0.60–1.00) | 0.30–3.20 | 0.4604 |
| BUN (mg/dL) | 8.0–22.0 | 18.5 (13.0–22.9) | 7.0–59.0 | 17.0 (12.0–22.0) | 10.0–53.0 | 0.2826 |
| Creatinine (mg/dL) | 0.65–1.07 | 0.82 (0.68–0.97) | 0.44–2.32 | 0.80 (0.64–0.95) | 0.41–3.80 | 0.7103 |
| eGFR (mL/min/1.73 m2) | >90.0 | 65.8 (52.2–77.0) | 21.9–140.9 | 64.6 (53.2–81.7) | 9.5–116.3 | 0.6110 |
| Sodium (mmol/L) | 138–145 | 140 (139–142) | 134–144 | 140 (139–142) | 130–147 | 0.9718 |
| Total cholesterol (mg/dL) | 150–199 | 170 (145–196) | 89–235 | 169 (145–206) | 124–267 | 0.9024 |
| Triglyceride (mg/dL) | 35–149 | 93 (66–125) | 29–305 | 99 (84–139) | 27–301 | 0.1285 |
| Creatine kinase (U/L) | 59–248 | 97 (66–147) | 17–264 | 82 (64–127) | 31–338 | 0.4887 |
| Glucose (mg/dL) | 0–99 | 110 (96–134) | 71–205 | 111 (99–149) | 82–304 | 0.3351 |
| HbA1c (%) | 4.3–5.8 | 6.1 (5.7–6.6) | 5.1–10.5 | 6.1 (5.7–6.8) | 4.7–9.3 | 0.7513 |
| Ammonia (µg/dL) | 13–86 | 52 (39–85) | 24–180 | 38 (30–65) | 13–130 | 0.0105 |
| Prothrombin activity (%) | 80–120 | 92 (84–105) | 30–130 | 97 (80–109) | 11–130 | 0.7333 |
| Hemoglobin (g/dL) | 13.7–16.8 | 12.5 (10.8–13.3) | 7.3–15.6 | 12.6 (10.6–13.5) | 8.1–15.2 | 0.7103 |
| White blood cell count (/µL) | 3300–8600 | 4200 (3500–5300) | 1000–8900 | 4700 (3300–5900) | 500–8700 | 0.4805 |
| Platelet count (× 103/mm3) | 15.8–34.8 | 132.0 (94.0–174.0) | 62.0–368.0 | 129.0 (90.0–178.0) | 46.0–365.0 | 0.9828 |
| Neutrophil/lymphocyte Ratio | 0.86–2.77 | 2.37 (1.68–3.36) | 0.62–11.2 | 2.44 (1.68–3.40) | 0.50–11.71 | 0.8568 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuchihashi, J.; Koya, S.; Hirota, K.; Koga, N.; Narao, H.; Tomita, M.; Kawaguchi, T.; Hashida, R.; Nakano, D.; Tsutsumi, T.; et al. Effects of In-Hospital Exercise on Frailty in Patients with Hepatocellular Carcinoma. Cancers 2021, 13, 194. https://doi.org/10.3390/cancers13020194
Tsuchihashi J, Koya S, Hirota K, Koga N, Narao H, Tomita M, Kawaguchi T, Hashida R, Nakano D, Tsutsumi T, et al. Effects of In-Hospital Exercise on Frailty in Patients with Hepatocellular Carcinoma. Cancers. 2021; 13(2):194. https://doi.org/10.3390/cancers13020194
Chicago/Turabian StyleTsuchihashi, Jin, Shunji Koya, Keisuke Hirota, Noboru Koga, Hayato Narao, Manabu Tomita, Takumi Kawaguchi, Ryuki Hashida, Dan Nakano, Tsubasa Tsutsumi, and et al. 2021. "Effects of In-Hospital Exercise on Frailty in Patients with Hepatocellular Carcinoma" Cancers 13, no. 2: 194. https://doi.org/10.3390/cancers13020194
APA StyleTsuchihashi, J., Koya, S., Hirota, K., Koga, N., Narao, H., Tomita, M., Kawaguchi, T., Hashida, R., Nakano, D., Tsutsumi, T., Yoshio, S., Matsuse, H., Sanada, T., Notsumata, K., & Torimura, T. (2021). Effects of In-Hospital Exercise on Frailty in Patients with Hepatocellular Carcinoma. Cancers, 13(2), 194. https://doi.org/10.3390/cancers13020194