Nutritional Status as a Predictive Biomarker for Immunotherapy Outcomes in Advanced Head and Neck Cancer
Abstract
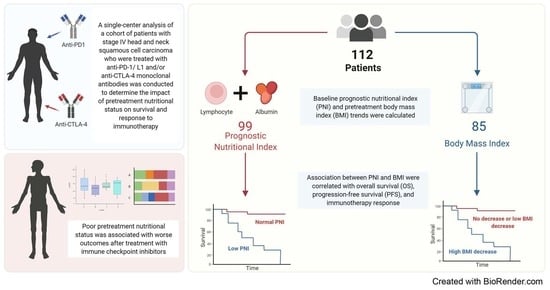
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Evaluation of Baseline PNI Score and Pretreatment BMI Trends
2.3. Statistical Analysis
3. Results
3.1. Analysis of Baseline Characteristics and Survival Outcomes
3.2. Relationship between PNI, BMI, and Immunotherapy Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Vigneswaran, N.; Williams, M.D. Epidemiological Trends in Head and Neck Cancer and Aids in Diagnosis. Oral. Maxillofac. Surg. Clin. N. Am. 2014, 26, 123–141. [Google Scholar] [CrossRef]
- Konings, H.; Stappers, S.; Geens, M.; De Winter, B.Y.; Lamote, K.; van Meerbeeck, J.P.; Specenier, P.; Vanderveken, O.M.; Ledeganck, K.J. A Literature Review of the Potential Diagnostic Biomarkers of Head and Neck Neoplasms. Front. Oncol. 2020, 10, 1020. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Sim, F.; Leidner, R.; Bell, R.B. Immunotherapy for Head and Neck Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Gavrielatou, N.; Doumas, S.; Economopoulou, P.; Foukas, P.G.; Psyrri, A. Biomarkers for immunotherapy response in head and neck cancer. Cancer Treat. Rev. 2020, 84, 101977. [Google Scholar] [CrossRef]
- Nozoe, T.; Kimura, Y.; Ishida, M.; Saeki, H.; Korenaga, D.; Sugimachi, K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur. J. Surg. Oncol. 2002, 28, 396–400. [Google Scholar] [CrossRef]
- Bruixola, G.; Caballero, J.; Papaccio, F.; Petrillo, A.; Iranzo, A.; Civera, M.; Moriana, M.; Bosch, N.; Maroñas, M.; González, I.; et al. Prognostic Nutritional Index as an independent prognostic factor in locoregionally advanced squamous cell head and neck cancer. ESMO Open 2018, 3, e000425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar] [PubMed]
- Tsai, M.H.; Huang, T.L.; Chuang, H.C.; Lin, Y.T.; Fang, F.M.; Lu, H.; Chien, C.Y. Clinical significance of pretreatment prognostic nutritional index and lymphocyte-to-monocyte ratio in patients with advanced p16-negative oropharyngeal cancer—A retrospective study. PeerJ 2020, 8, e10465. [Google Scholar] [CrossRef] [PubMed]
- Fanetti, G.; Polesel, J.; Fratta, E.; Muraro, E.; Lupato, V.; Alfieri, S.; Gobitti, C.; Minatel, E.; Matrone, F.; Caroli, A.; et al. Prognostic Nutritional Index Predicts Toxicity in Head and Neck Cancer Patients Treated with Definitive Radiotherapy in Association with Chemotherapy. Nutrients 2021, 13, 1277. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Xiao, W.; Wang, L.; Han, F.; Wu, H.; Deng, X.; Guo, X.; Zhao, C. The value of the Prognostic Nutritional Index (PNI) in predicting outcomes and guiding the treatment strategy of nasopharyngeal carcinoma (NPC) patients receiving intensity-modulated radiotherapy (IMRT) with or without chemotherapy. J. Cancer Res. Clin. Oncol. 2017, 143, 1263–1273. [Google Scholar] [CrossRef]
- Ling, H.H.; Yeh, K.Y.; Ng, S.H.; Wang, C.H.; Lai, C.H.; Wu, T.H.; Chang, P.H.; Chou, W.C.; Chen, F.P.; Lin, Y.C. Determining Malnutrition Assessment Criteria to Predict One-Year Mortality for Locally Advanced Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy. Nutrients 2020, 12, 836. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.H.; Hsieh, J.C.; Yeh, K.Y.; Chen, E.Y.; Yang, S.W.; Huang, J.S.; Lai, C.H.; Wu, T.H.; Huang, Y.M.; Chang, Y.S.; et al. Prognostic nutritional index relevance in chemoradiotherapy for advanced oral cavity, oropharyngeal and hypopharyngeal cancer. Asia Pac. J. Clin. Nutr. 2018, 27, 996–1001. [Google Scholar] [CrossRef]
- Shoji, F.; Takeoka, H.; Kozuma, Y.; Toyokawa, G.; Yamazaki, K.; Ichiki, M.; Takeo, S. Pretreatment prognostic nutritional index as a novel biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer 2019, 136, 45–51. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Liu, F.; Qiu, X.; Zhang, X.; Fang, C.; Qian, X.; Li, Y. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol. Immunother. 2020, 69, 1813–1822. [Google Scholar] [CrossRef]
- Richtig, G.; Hoeller, C.; Wolf, M.; Wolf, I.; Rainer, B.M.; Schulter, G.; Richtig, M.; Grübler, M.R.; Gappmayer, A.; Haidn, T.; et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: An observational multi-centre study. PLoS ONE 2018, 13, e0204729. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Kichenadasse, G.; Miners, J.O.; Mangoni, A.A.; Rowland, A.; Hopkins, A.M.; Sorich, M.J. Association between Body Mass Index and Overall Survival with Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2020, 6, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Ricciuti, B.; Tiseo, M.; Bria, E.; Banna, G.L.; Aerts, J.G.; Barbieri, F.; Giusti, R.; Cortinovis, D.L.; Migliorino, M.R.; et al. Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD-L1 expression ≥50%: A multicenter study with external validation. J. Immunother. Cancer 2020, 8, e001403. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Indini, A.; De Luca, M.; Merelli, B.; Mariuk-Jarema, A.; Teterycz, P.; Rogala, P.; Lugowska, I.; Cybulska-Stopa, B.; Labianca, A.; et al. Body mass index (BMI) and outcome of metastatic melanoma patients receiving targeted therapy and immunotherapy: A multicenter international retrospective study. J. Immunother. Cancer 2020, 8, e001117. [Google Scholar] [CrossRef] [PubMed]
- Young, A.C.; Quach, H.T.; Song, H.; Davis, E.J.; Moslehi, J.J.; Ye, F.; Williams, G.R.; Johnson, D.B. Impact of body composition on outcomes from anti-PD1 +/− anti-CTLA-4 treatment in melanoma. J. Immunother. Cancer 2020, 8, e000821. [Google Scholar] [CrossRef] [PubMed]
- Magri, V.; Gottfried, T.; Di Segni, M.; Urban, D.; Peled, M.; Daher, S.; Stoff, R.; Bar, J.; Onn, A. Correlation of body composition by computerized tomography and metabolic parameters with survival of nivolumab-treated lung cancer patients. Cancer Manag. Res. 2019, 11, 8201–8207. [Google Scholar] [CrossRef] [Green Version]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The Obesity Paradox in Cancer: A Review. Curr. Oncol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef]
- Johannet, P.; Sawyers, A.; Qian, Y.; Kozloff, S.; Gulati, N.; Donnelly, D.; Zhong, J.; Osman, I. Baseline prognostic nutritional index and changes in pretreatment body mass index associate with immunotherapy response in patients with advanced cancer. J. Immunother. Cancer 2020, 8, e001674. [Google Scholar] [CrossRef]
- Chang, C.H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [Green Version]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef] [Green Version]
- Kedia-Mehta, N.; Finlay, D.K. Competition for nutrients and its role in controlling immune responses. Nat. Commun. 2019, 10, 2123. [Google Scholar] [CrossRef]
- Cohen, S.; Danzaki, K.; MacIver, N.J. Nutritional effects on T-cell immunometabolism. Eur. J. Immunol. 2017, 47, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Indini, A.; Rijavec, E.; Ghidini, M.; Tomasello, G.; Cattaneo, M.; Barbin, F.; Bareggi, C.; Galassi, B.; Gambini, D.; Grossi, F. Impact of BMI on Survival Outcomes of Immunotherapy in Solid Tumors: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 2628. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef]
- Sanchez, A.; Furberg, H. Comments on: Obesity Paradox in Patients with Non-Small Cell Lung Cancer Treated with Immunotherapy. JAMA Oncol. 2020, 6, 940–941. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef]
- Mirili, C.; Yılmaz, A.; Demirkan, S.; Bilici, M.; Basol Tekin, S. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int. J. Clin. Oncol. 2019, 24, 1301–1310. [Google Scholar] [CrossRef]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? J. Parenter. Enteral. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Ottery, F.D. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996, 12 (Suppl. S1), S15–S19. [Google Scholar] [CrossRef]



| Characteristic | N = 99 1 |
|---|---|
| Age | 64 (57, 70) |
| Sex | |
| Female | 13 (13%) |
| Male | 86 (87%) |
| Smoking Status | |
| Active | 1 (1.0%) |
| Current | 6 (6.1%) |
| Former | 55 (56%) |
| Never | 37 (37%) |
| Alcohol History | |
| No | 72 (73%) |
| Yes | 22 (22%) |
| Quit | 4 (4.0%) |
| Unknown | 1 (1.0%) |
| T Stage | |
| T1–2 | 42 (42%) |
| T3–4 | 40 (40%) |
| Unknown | 17 (17%) |
| N Stage | |
| N0–1 | 24 (24%) |
| N2–3 | 57 (58%) |
| Unknown | 18 (18%) |
| M Stage | |
| M0 | 15 (15%) |
| M1 | 66 (67%) |
| Unknown | 18 (18%) |
| Tumor Grade | |
| Well differentiated | 7 (7.1%) |
| Moderately differentiated | 19 (19%) |
| Poorly differentiated | 46 (46%) |
| Undifferentiated | 9 (9.1%) |
| Unknown | 18 (18%) |
| ECOG Score | |
| 0 | 4 (4.0%) |
| 1 | 89 (90%) |
| 2 | 6 (6.1%) |
| HPV Status | |
| Negative | 23 (23%) |
| Positive | 39 (39%) |
| Unknown | 37 (37%) |
| Primary Site | |
| Non-oropharynx | 58 (59%) |
| Oropharynx | 41 (41%) |
| PNI in baseline | 45.8 (42.8, 49.0) |
| BMI in baseline | 25.0 (22.1, 28.5) |
| BMI pretreatment trend | 0.02 (−0.02, 0.07) |
| IO Name | |
| Anti-PD-L1 | 2 (2.0%) |
| Anti-PD1 | 88 (89%) |
| Anti-PD1 and anti-CTLA4 | 9 (9.1%) |
| Previous Treatment | |
| Surgery, chemotherapy, and radiotherapy | 44 (44%) |
| Surgery and radiotherapy | 4 (4%) |
| Chemotherapy and radiotherapy | 46 (46%) |
| Chemotherapy alone | 1 (1%) |
| Radiotherapy alone | 2 (2%) |
| Unknown | 2 (2%) |
| Overall Survival | Progression-Free Survival | |||
|---|---|---|---|---|
| Characteristic | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age | 0.988 (0.96–0.999) | 0.043 | 0.981 (0.967–0.996) | 0.016 |
| Sex | ||||
| Male vs. Female | 1.87 (0.741–4.71) | 0.185 | 1.22 (0.649–2.3) | 0.533 |
| Smoking Status | ||||
| Current vs. Active | 0.59 (0.053–6.56) | 0.668 | 1.42 (0.165–12.2) | 0.748 |
| Former vs. Active | 1.04 (0.14–7.63) | 0.972 | 1.83 (0.251–13.3) | 0.551 |
| Never vs. Active | 0.68 (0.09–5.19) | 0.714 | 1.65 (0.224–12.1) | 0.624 |
| Alcohol History | ||||
| Yes vs. No | 0.493 (0.23–1.05) | 0.0679 | 0.734 (0.436–1.24) | 0.247 |
| Quit vs. No | 1.14 (0.353–3.7) | 0.823 | 0.622 (0.195–1.98) | 0.422 |
| T Stage | ||||
| T3–4 vs. T1–2 | 1.26 (0.688–2.32) | 0.451 | 0.911 (0.571–1.46) | 0.698 |
| N Stage | ||||
| N2–3 vs. N0–1 | 0.873 (0.435–1.75) | 0.703 | 0.754 (0.454–1.25) | 0.274 |
| M Stage | ||||
| M1 vs. M0 | 2.2 (0.856–5.68) | 0.101 | 1.65 (0.837–3.24) | 0.148 |
| Tumor Grade | ||||
| Moderately vs. Well | 0.66 (0.203–2.15) | 0.491 | 0.68 (0.263–1.76) | 0.427 |
| Poorly vs. Well | 0.721 (0.248–2.09) | 0.547 | 0.632 (0.267–1.49) | 0.296 |
| Undifferentiated vs. Well | 0.646 (0.161–2.59) | 0.537 | 0.722 (0.249–2.09) | 0.549 |
| ECOG Score | ||||
| 1 vs. 0 | 1.01 (0.456–2.24) | 0.982 | 1.34 (0.765–2.35) | 0.305 |
| HPV Status | ||||
| Positive vs. Negative | 1.29 (0.613–2.71) | 0.504 | 0.867 (0.501–1.5) | 0.61 |
| Primary Site | ||||
| Oropharynx vs. non-Oropharynx | 1.23 (0.709–2.13) | 0.464 | 1.02 (0.667–1.55) | 0.937 |
| IO Name | ||||
| Anti-PD-L1 vs. anti-PD1 | 3.72 × 10−8 (0-Inf) | 0.996 | 0.206 (0.0285–1.5) | 0.118 |
| Anti-PD1 and anti-CTLA4 vs. anti-PD1 | 0.801 (0.317–2.02) | 0.637 | 0.952 (0.458–1.98) | 0.894 |
| PNI Baseline Group | ||||
| Normal (≥45) vs. Low (<45) | 0.464 (0.265–0.814) | 0.007 | 0.525 (0.341–0.808) | 0.003 |
| BMI Baseline Group | ||||
| Obese (n = 16) vs. normal (n = 49) | 0.463 (0.189–1.13) | 0.091000 | 1.08 (0.598–1.94) | 0.805000 |
| Overweight (n = 34) vs. normal (n = 49) | 0.793 (0.438–1.44) | 0.445000 | 0.709 (0.441–1.14) | 0.157000 |
| BMI Pretreatment Trend Group | ||||
| Decrease <2% vs. Decrease ≥2% | 0.42 (0.229–0.77) | 0.005 | 0.75 (0.474–1.19) | 0.221 |
| Overall Survival | Progression-Free Survival | |||||
|---|---|---|---|---|---|---|
| Characteristic | Log (HR) 1 | 95% CI 1 | p Value | Log (HR) 1 | 95% CI 1 | p Value |
| Age | −0.02 | −0.06, 0.01 | 0.2 | −0.03 | −0.06, 0.00 | 0.040 |
| Sex | ||||||
| Female | - | - | - | - | ||
| Male | 0.15 | −1.0, 1.3 | 0.8 | 0.40 | −0.40, 1.2 | 0.3 |
| T Stage | ||||||
| T1–2 | - | - | - | - | ||
| T3–4 | 0.32 | −0.42, 1.0 | 0.4 | 0.27 | −0.30, 0.83 | 0.4 |
| Unknown | ||||||
| N Stage | ||||||
| N0–1 | - | - | - | - | ||
| N2–3 | −0.28 | −1.1, 0.58 | 0.5 | −0.31 | −1.0, 0.34 | 0.4 |
| Unknown | −0.16 | −1.7, 1.4 | 0.8 | 0.23 | −1.2, 1.7 | 0.7 |
| HPV Status | ||||||
| Negative | - | - | - | - | ||
| Positive | −0.30 | −1.8, 1.2 | 0.7 | −0.41 | −1.5, 0.65 | 0.4 |
| Unknown | −0.03 | −1.2, 1.2 | >0.9 | −0.71 | −1.7, 0.26 | 0.2 |
| Primary Site | ||||||
| Non-oropharyngeal | - | - | - | - | ||
| Oropharyngeal | 0.80 | −0.49, 2.1 | 0.2 | 0.08 | −0.87, 1.0 | 0.9 |
| PNI Group | ||||||
| Low | - | - | - | - | ||
| Normal | −0.79 | −1.6, −0.03 | 0.041 | −0.78 | −1.4, −0.18 | 0.011 |
| Pretreatment BMI Trend | ||||||
| Decrease ≥2% | - | - | - | - | ||
| Decrease <2% | −0.50 | −1.3, 0.34 | 0.2 | 0.36 | −0.35, 1.1 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guller, M.; Herberg, M.; Amin, N.; Alkhatib, H.; Maroun, C.; Wu, E.; Allen, H.; Zheng, Y.; Gourin, C.; Vosler, P.; et al. Nutritional Status as a Predictive Biomarker for Immunotherapy Outcomes in Advanced Head and Neck Cancer. Cancers 2021, 13, 5772. https://doi.org/10.3390/cancers13225772
Guller M, Herberg M, Amin N, Alkhatib H, Maroun C, Wu E, Allen H, Zheng Y, Gourin C, Vosler P, et al. Nutritional Status as a Predictive Biomarker for Immunotherapy Outcomes in Advanced Head and Neck Cancer. Cancers. 2021; 13(22):5772. https://doi.org/10.3390/cancers13225772
Chicago/Turabian StyleGuller, Meytal, Matthew Herberg, Neha Amin, Hosam Alkhatib, Christopher Maroun, Evan Wu, Hailey Allen, Ying Zheng, Christine Gourin, Peter Vosler, and et al. 2021. "Nutritional Status as a Predictive Biomarker for Immunotherapy Outcomes in Advanced Head and Neck Cancer" Cancers 13, no. 22: 5772. https://doi.org/10.3390/cancers13225772
APA StyleGuller, M., Herberg, M., Amin, N., Alkhatib, H., Maroun, C., Wu, E., Allen, H., Zheng, Y., Gourin, C., Vosler, P., Tan, M., Koch, W., Eisele, D., Seiwert, T., Fakhry, C., Pardoll, D., Zhu, G., & Mandal, R. (2021). Nutritional Status as a Predictive Biomarker for Immunotherapy Outcomes in Advanced Head and Neck Cancer. Cancers, 13(22), 5772. https://doi.org/10.3390/cancers13225772