Oxidative Distress Induces Wnt/β-Catenin Pathway Modulation in Colorectal Cancer Cells: Perspectives on APC Retained Functions
Abstract
:Simple Summary
Abstract
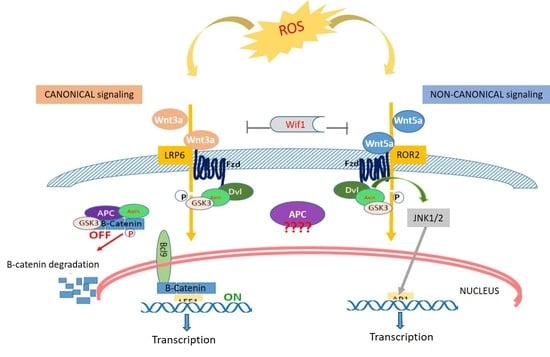
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Treatments
2.2. Cell Viability and Metabolic Assay
2.3. Gene Expression by Real-Time Quantitative PCR Analysis (qRT-PCR)
| 5′-GCTTGATAGCTACAAATGAGGACC-3′ and 5′-CCACAAAGTTCCACATGC-3′ |
| for APC; RefSeq: [NM_000038] |
| 5′-CCCATGCACCTGGTTCTACT-3′ and 5′-CCAAGCCACAGGGATACAGT-3′ |
| for LRP-6; RefSeq: [NM_002336] |
| 5′-ATGGGTTCTGCCAGCCTTAC-3′ and 5′-TAGACGTGCCGATCATGGTG-3′ |
| for ROR-2; RefSeq: [NM_004560] |
| 5′-CATGAACCGCCACAACAAC-3′ and 5′-TGGCACTTGCACTTGAGGT-3′ |
| for WNT-3a; RefSeq: [NM_033131] |
| 5′-CTCATGAACCTGCACAACAACG-3′ and 5′-CCAGCATGTCTTCAGGCTACAT-3′ |
| for WNT-5a; RefSeq: [NM_03392] |
| 5′-CCAACTTGCCATCAATGAATAA-3′ and 5′-GGCATCTGATTGGAGTGAGAA-3′ |
| for BCL-9; RefSeq: [NM_004326] |
| 5′-GAC GAG ATG ATC CCC TTC AA-3′ and 5′-AGG GCT CCT GAG AGG TTT GT-3′ |
| for LEF-1; RefSeq: [NM_016269] |
| 5′-TCGACATGGAGTCCCAGGA-3′ and 5-GGCGATTCTCTCCAGCTTCC-3′ |
| for JUN/AP-1; RefSeq: [NM_002228] |
| 5′-AGCCAGTTCCTCATCAATGG-3′ and 5′-GGTAGTGGCTGGTACGGAAA-3′ |
| for GUSB; RefSeq: [NM_000181] |
2.4. Flow Cytometry and Cell Cycle Assay
2.5. Wound Healing
2.6. Preparing Cell Blocks from HCT116, SW480 and SW620 Cell Lines and Performing the Immunocytochemical (ICC) Stainings
2.7. Western Blotting
2.8. Statistical Analysis and Tools
3. Results
3.1. Cell Viability after Oxidative Distress Induced by H2O2
3.2. Gene Expression Analysis by qPCR Real Time
3.3. Gene Expression Cluster Analysis
3.4. Cell Migration under Oxidative Distress by H2O2 [0.05 mM]
3.5. Cell Cycle Analysis
3.6. Protein Expression Assay by Western Blotting
3.7. Expression of Wnt Pathway Components in HCT116, SW480 and SW620 Cell Block Sections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP1 | Activator protein-1 |
| APC | Adenomatous Polyposis Coli |
| BCL9 | B-cell CLL/lymphoma 9 protein |
| CF/LEF | T cell factor/lymphoid enhancer factor |
| CIN | Chromosome instability |
| CIMP | CpG island methylator phenotype |
| CRC | Colorectal Cancer |
| FACS | fluorescence-activated cell sorting |
| FZ-6 | Frizzled-6 |
| GUSB | Beta-glucuronidase |
| ICC | Immunocytochemistry |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LRP6 | Low-density lipoprotein receptor-related protein 6 |
| MHL1 | MutL homolog 1 |
| MMR | DNA mismatch repair |
| MSI | microsatellite instability |
| MSS | microsatellite stability; |
| MTS | 2-[2-methoxy-4-nitrophenyl]-3-[4-nitrophenyl]-5-[2,4-disulphophenyl]-2H-tetrazolium, monosodium salt |
| KRAS | GTPase KRas |
| ROS | reactive oxygen species |
| ROR2 | Receptor Tyrosine Kinase Like Orphan Receptor 2 |
| WIF1 | WNT Inhibitory Factor 1 |
| WNT | Wingless/It |
| WNT3a | Wnt Family Member 3A |
| WNT5a | Wnt Family Member 5A |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Conti, L.; Del Cornò, M.; Gessani, S. Revisiting the impact of lifestyle on colorectal cancer risk in a gender perspective. Crit. Rev. Oncol. Hematol. 2020, 145, 102834. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Beluşică, L.; Stavarachi, M.; Apostol, P.; Spandole, S.; Radu, I.; Cimponeriu, D. Rating the environmental and genetic risk factors for colorectal cancer. J. Med. Life 2012, 5, 152–159. [Google Scholar] [PubMed]
- Aceto, G.M.; Catalano, T.; Curia, M.C. Molecular Aspects of Colorectal Adenomas: The Interplay among Microenvironment, Oxidative Stress, and Predisposition. Biomed. Res. Int. 2020, 2020, 1726309. [Google Scholar] [CrossRef] [PubMed]
- Sugai, T.; Takahashi, Y.; Eizuka, M.; Sugimoto, R.; Fujita, Y.; Habano, W.; Otsuka, K.; Sasaki, A.; Yamamoto, E.; Matsumoto, T.; et al. profiling and genome-wide analysis based on somatic copy number alterations in advanced colorectal cancers. Mol. Carcinog. 2018, 57, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Divya, T.; Kumar, K.; Dineshbabu, V.; Velavan, B.; Sudhandiran, G. Colorectal carcinogenesis: Insights into the cell death and signal transduction pathways: A review. World J. Gastrointest. Oncol. 2018, 10, 244–259. [Google Scholar]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef]
- Schatoff, E.M.; Leach, B.I.; Dow, L.E. Wnt Signaling and Colorectal Cancer. Curr. Colorectal Cancer Rep. 2017, 13, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef]
- Novellasdemunt, L.; Antas, P.; Li, V.S. Targeting Wnt signaling in colorectal cancer. A Review in the Theme: Cell Signaling: Proteins, Pathways and Mechanisms. Am. J. Physiol. Cell. Physiol. 2015, 309, C511–C521. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najdi, R.; Holcombe, R.F.; Waterman, M.L. Wnt signaling and colon carcinogenesis: Beyond APC. J. Carcinog. 2011, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Lecarpentier, Y. Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis. Front Immunol. 2018, 9, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanderVorst, K.; Dreyer, C.A.; Konopelski, S.E.; Lee, H.; Ho, H.H.; Carraway, K.L., 3rd. Wnt/PCP Signaling Contribution to Carcinoma Collective Cell Migration and Metastasis. Cancer Res. 2019, 79, 1719–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuriaga, M.A.; Fuster, J.J.; Farb, M.G.; MacLauchlan, S.; Bretón-Romero, R.; Karki, S.; Hess, D.T.; Apovian, C.M.; Hamburg, N.M.; Gokce, N.; et al. Activation of non-canonical WNT signaling in human visceral adipose tissue contributes to local and systemic inflammation. Sci. Rep. 2017, 7, 17326. [Google Scholar] [CrossRef] [PubMed]
- Pashirzad, M.; Shafiee, M.; Rahmani, F.; Behnam-Rassouli, R.; Hoseinkhani, F.; Ryzhikov, M.; Moradi Binabaj, M.; Parizadeh, M.R.; Avan, A.; Hassanian, S.M. Role of Wnt5a in the Pathogenesis of Inflammatory Diseases. J. Cell Physiol. 2017, 232, 1611–1616. [Google Scholar] [CrossRef]
- Zhang, C.; Tannous, E.; Zheng, J.J. Oxidative stress upregulates Wnt signaling in human retinal microvascular endothelial cells through activation of disheveled. J. Cell Biochem. 2019, 120, 14044–14054. [Google Scholar] [CrossRef]
- Perše, M. Oxidative stress in the pathogenesis of colorectal cancer: Cause or consequence? Biomed. Res. Int. 2013, 2013, 725710. [Google Scholar] [CrossRef] [Green Version]
- Andrisic, L.; Dudzik, D.; Barbas, C.; Milkovic, L.; Grune, T.; Zarkovic, N. Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol. 2018, 14, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Marjaneh, R.; Hassanian, S.M.; Mehramiz, M.; Rezayi, M.; Ferns, G.A.; Khazaei, M.; Avan, A. Reactive oxygen species in colorectal cancer: The therapeutic impact and its potential roles in tumor progression via perturbation of cellular and physiological dysregulated pathways. J. Cell Physiol. 2019, 234, 10072–10079. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, Y.; Zamyatnin, A.A., Jr.; Werner, J.; Bazhin, A.V. Reactive oxygen species and colorectal cancer. J. Cell. Physiol. 2018, 233, 5119–5132. [Google Scholar] [CrossRef] [PubMed]
- Carini, F.; Mazzola, M.; Rappa, F.; Jurjus, A.; Geagea, A.G.; Al Kattar, S.; Bou-Assi, T.; Jurjus, R.; Damiani, P.; Leone, A.; et al. Colorectal Carcinogenesis: Role of Oxidative Stress and Antioxidants. Anticancer Res. 2017, 37, 4759–4766. [Google Scholar] [PubMed] [Green Version]
- Babu, K.R.; Tay, Y. The Yin-Yang Regulation of Reactive Oxygen Species and MicroRNAs in Cancer. Int. J. Mol. Sci. 2019, 20, 5335. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Zhu, C.; Hu, W.; Wu, H.; Hu, X. No evident dose-response relationship between cellular ROS level and its cytotoxicity–a paradoxical issue in ROS-based cancer therapy. Sci Rep. 2014, 4, 5029. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug. Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Banskota, S.; Dahal, S.; Kwon, E.; Kim, D.Y.; Kim, J.A. β-Catenin gene promoter hypermethylation by reactive oxygen species correlates with the migratory and invasive potentials of colon cancer cells. Cell Oncol. 2018, 41, 569–580. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.H.; Yoo, B.C.; Ku, J.L. Induction of LGR5 by H2O2 treatment is associated with cell proliferation via the JNK signaling pathway in colon cancer cells. Int. J. Oncol. 2012, 41, 1744–1750. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Fu, Z.; Wang, H.; Feng, J.; Wei, J.; Guo, J. Peroxiredoxin 2 knockdown by RNA interference inhibits the growth of colorectal cancer cells by downregulating Wnt/β-catenin signaling. Cancer Lett. 2014, 343, 190–199. [Google Scholar] [CrossRef]
- Jaiswal, A.S.; Narayan, S. A novel function of adenomatous polyposis coli (APC) in regulating DNA repair. Cancer Lett. 2008, 271, 272–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, djw332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brocardo, M.; Lei, Y.; Tighe, A.; Taylor, S.S.; Mok, M.T.; Henderson, B.R. Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: Regulation of Bcl-2 and implications for cell survival. J. Biol. Chem. 2008, 283, 5950–5959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segditsas, S.; Rowan, A.J.; Howarth, K.; Jones, A.; Leedham, S.; Wright, N.A.; Gorman, P.; Chambers, W.; Domingo, E.; Roylance, R.R.; et al. APC and the three-hit hypothesis. Oncogene 2009, 28, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Pirkmajer, S.; Chibalin, A.V. Serum starvation: Caveat emptor. Am. J. Physiol. Cell Physiol. 2011, 301, C272–C279. [Google Scholar] [CrossRef] [Green Version]
- Lanuti, P.; Fuhrmann, S.; Lachmann, R.; Marchisio, M.; Miscia, S.; Kern, F. Simultaneous characterization of phospho-proteins and cell cycle in activated T cell subsets. Int. J. Immunopathol. Pharmacol. 2009, 22, 689–698. [Google Scholar] [CrossRef]
- Bologna, G.; Lanuti, P.; D’Ambrosio, P.; Tonucci, L.; Pierdomenico, L.; D’Emilio, C.; Celli, N.; Marchisio, M.; d’Alessandro, N.; Santavenere, E.; et al. Water-soluble platinum phthalocyanines as potential antitumor agents. Biometals 2014, 27, 575–589. [Google Scholar] [CrossRef]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef] [Green Version]
- COSMIC v94, the Catalogue of Somatic Mutation in Cancer, Released May 2021. Available online: https://cancer.sanger.ac.uk/cosmic (accessed on 26 July 2021).
- Tronov, V.A.; Kramarenko, I.I.; Maĭorova, E.N.; Zakharov, S.F. Mismatch repair (MMR) efficiency and MSH2 gene mutation in human colorectal carcinoma cell line COLO320HSR. Genetika 2007, 43, 537–544. [Google Scholar] [CrossRef]
- Hewitt, R.E.; McMarlin, A.; Kleiner, D.; Wersto, R.; Martin, P.; Tsokos, M.; Stamp, G.W.; Stetler-Stevenson, W.G. Validation of a model of colon cancer progression. J. Pathol. 2000, 192, 446–454. [Google Scholar] [CrossRef]
- Ilyas, M.; Tomlinson, I.P.; Rowan, A.; Pignatelli, M.; Bodmer, W.F. Beta-catenin mutations in cell lines established from human colorectal cancers. Proc. Natl. Acad. Sci. USA 1997, 94, 10330–10334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-omics of 34 colorectal cancer cell lines—A resource for biomedical studies. Mol. Cancer. 2017, 16, 116. [Google Scholar] [CrossRef]
- He, B.; Reguart, N.; You, L.; Mazieres, J.; Xu, Z.; Lee, A.Y.; Mikami, I.; McCormick, F.; Jablons, D.M. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene 2005, 24, 3054–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorham, Q.J.; Janssen, J.; Tijssen, M.; Snellenberg, S.; Mongera, S.; van Grieken, N.C.; Grabsch, H.; Kliment, M.; Rembacken, B.J.; Mulder, C.J.; et al. Promoter methylation of Wnt-antagonists in polypoid and nonpolypoid colorectal adenomas. BMC Cancer 2013, 13, 603. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell Longev. 2019, 12. [Google Scholar] [CrossRef]
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Küttner, V.; et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361, eaao4227. [Google Scholar] [CrossRef] [Green Version]
- Kirtonia, A.; Sethi, G.; Garg, M. The multifaceted role of reactive oxygen species in tumor-igenesis. Cell Mol. Life Sci. 2020, 77, 4459–4483. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide–production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 2015, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer. 2017, 8, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Senft, D.; Ronai, Z.E. Adaptive Stress Responses During Tumor Metastasis and Dormancy. Trends Cancer. 2016, 2, 429–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlos-Suárez, A.; Barriga, F.M.; Jung, P.; Iglesias, M.; Céspedes, M.V.; Rossell, D.; Sevillano, M.; Hernando-Momblona, X.; da Silva-Diz, V.; Muñoz, P.; et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 2011, 8, 511–524. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Haase, G.; Ben-Ze’ev, A. Wnt signaling in cancer stem cells and colon cancer metastasis. F1000Research 2016, 5, 699. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.A.; Park, J.H.; Rhyu, S.Y.; Oh, S.T.; Kang, W.K.; Kim, H.N. Wnt3a expression is associated with MMP-9 expression in primary tumor and metastatic site in recurrent or stage IV colorectal cancer. BMC Cancer 2014, 14, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duranton, B.; Holl, V.; Schneider, Y.; Carnesecchi, S.; Gossé, F.; Raul, F.; Seiler, N. Polyamine metabolism in primary human colon adenocarcinoma cells (SW480) and their lymph node metastatic derivatives (SW620). Amino Acids. 2003, 24, 63–72. [Google Scholar] [CrossRef]
- Pariente, J.A.; Camello, C.; Camello, P.J.; Salido, G.M. Release of calcium from mitochondrial and non-mitochondrial intracellular stores in mouse pancreatic acinar cells by hydrogen peroxide. J. Membr. Biol. 2001, 179, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Kakinuma, S.; Tokairin, Y.; Arai, M.; Kohno, H.; Wakabayashi, K.; Imaoka, T.; Ito, E.; Koike, M.; Uetake, H.; et al. Mild inflammation accelerates colon carcinogenesis in Mlh1-deficient mice. Oncology 2006, 71, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 149185. [Google Scholar] [CrossRef] [Green Version]
- Kadosh, E.; Snir-Alkalay, I.; Venkatachalam, A.; May, S.; Lasry, A.; Elyada, E.; Zinger, A.; Shaham, M.; Vaalani, G.; Mernberger, M.; et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature 2020, 586, 133–138. [Google Scholar] [CrossRef] [PubMed]















| Antibody | Company/Catalog No. | Type (Clone) | Dilution (Incubation) |
|---|---|---|---|
| FZ-6 | Novus Biological/#NBP1-89702 | Rabbit polyclonal | 1:100 (ON) |
| APC | Thermo Fisher/PA530580 | Rabbit polyclonal | 1:100 (30′) |
| β-Catenin | BD Bio./610154 | Mouse monoclonal (Clone 14) | 1:3000 (60′) |
| E-cadherin | BD Bio./610181-82 | Mouse monoclonal (Clone 36) | 1:50 (30′) |
| Cyclin D1 | Ylem/MCP511 | Mouse monoclonal (P2D11F11) | 1:25 (60′) |
| Cells | MS | CIMP | CIN | WNT3a | WNT5a | LRP6 | WIF1 | ROR2 | APC | CTNNB1 | LEF1 | cJUN/AP1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCT116 | MSI | + | - | wt | wt | W419R | met | R302H | wt | S45del | wt | Wt |
| SW480 | MSS | - | + | wt | wt | wt | met | wt | Q1338 * | wt | wt | Wt |
| SW620 | MSS | - | + | wt | wt | wt | met | wt | Q1338 * | wt | wt | Wt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalano, T.; D’Amico, E.; Moscatello, C.; Di Marcantonio, M.C.; Ferrone, A.; Bologna, G.; Selvaggi, F.; Lanuti, P.; Cotellese, R.; Curia, M.C.; et al. Oxidative Distress Induces Wnt/β-Catenin Pathway Modulation in Colorectal Cancer Cells: Perspectives on APC Retained Functions. Cancers 2021, 13, 6045. https://doi.org/10.3390/cancers13236045
Catalano T, D’Amico E, Moscatello C, Di Marcantonio MC, Ferrone A, Bologna G, Selvaggi F, Lanuti P, Cotellese R, Curia MC, et al. Oxidative Distress Induces Wnt/β-Catenin Pathway Modulation in Colorectal Cancer Cells: Perspectives on APC Retained Functions. Cancers. 2021; 13(23):6045. https://doi.org/10.3390/cancers13236045
Chicago/Turabian StyleCatalano, Teresa, Emira D’Amico, Carmelo Moscatello, Maria Carmela Di Marcantonio, Alessio Ferrone, Giuseppina Bologna, Federico Selvaggi, Paola Lanuti, Roberto Cotellese, Maria Cristina Curia, and et al. 2021. "Oxidative Distress Induces Wnt/β-Catenin Pathway Modulation in Colorectal Cancer Cells: Perspectives on APC Retained Functions" Cancers 13, no. 23: 6045. https://doi.org/10.3390/cancers13236045
APA StyleCatalano, T., D’Amico, E., Moscatello, C., Di Marcantonio, M. C., Ferrone, A., Bologna, G., Selvaggi, F., Lanuti, P., Cotellese, R., Curia, M. C., Lattanzio, R., & Aceto, G. M. (2021). Oxidative Distress Induces Wnt/β-Catenin Pathway Modulation in Colorectal Cancer Cells: Perspectives on APC Retained Functions. Cancers, 13(23), 6045. https://doi.org/10.3390/cancers13236045