Long-Term Experience with Radiofrequency-Induced Hyperthermia Combined with Intravesical Chemotherapy for Non-Muscle Invasive Bladder Cancer
Abstract
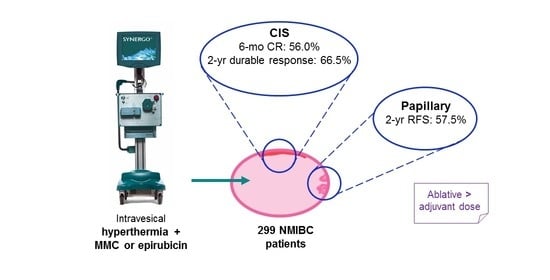
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. Efficacy
2.2.1. Complete Response
2.2.2. Durable Response and Recurrence-Free Survival
2.2.3. Progression
2.2.4. Overall Survival, Relative Survival and Cancer Specific Survival
2.2.5. Treatment after RF-CHT
2.2.6. Outcome after “Completed” RF-CHT Treatment
2.3. Tolerability and Safety
3. Discussion
4. Materials and Methods
4.1. Dataset Characteristics
4.2. Treatment Schedule and Follow-Up
4.3. Outcomes
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- GLOBOCAN. Estimated Cancer Incidence, Mortality, and Prevalence Worldwide in 2018. Available online: http://globocan.iarc.fr (accessed on 26 August 2020).
- Mossanen, M.; Gore, J.L. The burden of bladder cancer care: Direct and indirect costs. Curr. Opin. Urol. 2014, 24, 487–491. [Google Scholar] [CrossRef]
- Leal, J.; Luengo-Fernandez, R.; Sullivan, R.; Witjes, J.A. Economic Burden of Bladder Cancer across the European Union. Eur. Urol. 2016, 69, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Babjuk, M.; Burger, M.; Compérat, E.; Gontero, P.; Mostafid, A.H.; Palou, J.; Van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. EAU Guideline Non-Muscle-Invasive Bladder Cancer. In Proceedings of the EAU Annual Congress Amsterdam 2020, Virtual Congress, 17–21 July 2020; Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 26 August 2020).
- Guallar-Garrido, S.; Julián, E. Bacillus Calmette-Guérin (BCG) Therapy for Bladder Cancer: An Update. Immunotargets Ther. 2020, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cambier, S.; Sylvester, R.J.; Collette, L.; Gontero, P.; Brausi, M.A.; van Andel, G.; Kirkels, W.J.; Silva, F.C.; Oosterlinck, W.; Prescott, S.; et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guérin. Eur. Urol. 2016, 69, 60–69. [Google Scholar] [CrossRef]
- Aziz, A.; May, M.; Burger, M.; Palisaar, R.J.; Trinh, Q.D.; Fritsche, H.M.; Rink, M.; Chun, F.; Martini, T.; Bolenz, C.; et al. Prediction of 90-day mortality after radical cystectomy for bladder cancer in a prospective European multicenter cohort. Eur. Urol. 2014, 66, 156–163. [Google Scholar] [CrossRef]
- Nielsen, M.E.; Mallin, K.; Weaver, M.A.; Palis, B.; Stewart, A.; Winchester, D.P.; Milowsky, M.I. Association of hospital volume with conditional 90-day mortality after cystectomy: An analysis of the National Cancer Data Base. BJU Int. 2014, 114, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Hautmann, R.E.; de Petriconi, R.C.; Volkmer, B.G. Lessons learned from 1000 neobladders: The 90-day complication rate. J. Urol. 2010, 184, 990–994. [Google Scholar] [CrossRef]
- Hautmann, R.E.; Abol-Enein, H.; Davidsson, T.; Gudjonsson, S.; Hautmann, S.H.; Holm, H.V.; Lee, C.T.; Liedberg, F.; Madersbacher, S.; Manoharan, M.; et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Urinary diversion. Eur. Urol. 2013, 63, 67–80. [Google Scholar] [CrossRef]
- Smith, A.B.; Jaeger, B.; Pinheiro, L.C.; Edwards, L.J.; Tan, H.J.; Nielsen, M.E.; Reeve, B.B. Impact of bladder cancer on health-related quality of life. BJU Int. 2018, 121, 549–557. [Google Scholar] [CrossRef]
- Parker, W.P.; Smelser, W.; Lee, E.K.; Habermann, E.B.; Thapa, P.; Zaid, H.B.; Frank, I.; Griebling, T.L.; Tollefson, M.K.; Thompson, R.H.; et al. Utilization and Outcomes of Radical Cystectomy for High-grade Non-muscle-invasive Bladder Cancer in Elderly Patients. Clin. Genitourin. Cancer 2017, 16, e79–e97. [Google Scholar] [CrossRef]
- Van Valenberg, H.; Colombo, R.; Witjes, F. Intravesical radiofrequency-induced hyperthermia combined with chemotherapy for non-muscle-invasive bladder cancer. Int. J. Hyperth. 2016, 32, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Mantso, T.; Goussetis, G.; Franco, R.; Botaitis, S.; Pappa, A.; Panayiotidis, M. Effects of hyperthermia as a mitigation strategy in DNA damage-based cancer therapies. Semin. Cancer Biol. 2016, 37–38, 96–105. [Google Scholar] [CrossRef]
- Tan, W.S.; Kelly, J.D. Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat. Rev. Urol. 2018, 15, 667–685. [Google Scholar] [CrossRef]
- Van Valenberg, F.J.P.; van der Heijden, A.G.; Lammers, R.J.M.; Falke, J.; Arends, T.J.H.; Oosterwijk, E.; Witjes, J.A. Intravesical radiofrequency induced hyperthermia enhances mitomycin C accumulation in tumour tissue. Int. J. Hyperth. 2017, 34, 988–993. [Google Scholar] [CrossRef]
- Van der Heijden, A.G.; Verhaegh, G.; Jansen, C.F.; Schalken, J.A.; Witjes, J.A. Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: An in vitro study. J. Urol. 2005, 173, 1375–1380. [Google Scholar] [CrossRef]
- Tan, W.S.; Panchal, A.; Buckley, L.; Devall, A.J.; Loubière, L.S.; Pope, A.M.; Feneley, M.R.; Cresswell, J.; Issa, R.; Mostafid, H.; et al. Radiofrequency-induced Thermo-chemotherapy Effect Versus a Second Course of Bacillus Calmette-Guérin or Institutional Standard in Patients with Recurrence of Non-muscle-invasive Bladder Cancer Following Induction or Maintenance Bacillus Calmette-Guérin Therapy (HYMN): A Phase III, Open-label, Randomised Controlled Trial. Eur. Urol. 2019, 75, 63–71. [Google Scholar] [CrossRef]
- Arends, T.J.; Nativ, O.; Maffezzini, M.; de Cobelli, O.; Canepa, G.; Verweij, F.; Moskovitz, B.; van der Heijden, A.G.; Witjes, J.A. Results of a Randomised Controlled Trial Comparing Intravesical Chemohyperthermia with Mitomycin C Versus Bacillus Calmette-Guerin for Adjuvant Treatment of Patients with Intermediate- and High-risk Non-Muscle-invasive Bladder Cancer. Eur. Urol. 2016, 69, 1046–1052. [Google Scholar] [CrossRef]
- Gofrit, O.N.; Shapiro, A.; Pode, D.; Sidi, A.; Nativ, O.; Leib, Z.; Witjes, J.A.; van der Heijden, A.G.; Naspro, R.; Colombo, R. Combined local bladder hyperthermia and intravesical chemotherapy for the treatment of high-grade superficial bladder cancer. Urology 2004, 63, 466–471. [Google Scholar] [CrossRef]
- Arends, T.J.; van der Heijden, A.G.; Witjes, J.A. Combined chemohyperthermia: 10-year single center experience in 160 patients with nonmuscle invasive bladder cancer. J. Urol. 2014, 192, 708–713. [Google Scholar] [CrossRef]
- de Jong, J.J.; Hendricksen, K.; Rosier, M.; Mostafid, H.; Boormans, J.L. Hyperthermic Intravesical Chemotherapy for BCG Unresponsive Non-Muscle Invasive Bladder Cancer Patients. Bladder Cancer 2018, 4, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Van Valenberg, F.J.P.; Kajtazovic, A.; Canepa, G.; Lüdecke, G.; Kilb, J.I.; Aben, K.K.H.; Nativ, O.; Madaan, S.; Ayres, B.; Issa, R.; et al. Intravesical Radiofrequency-Induced Chemohyperthermia for Carcinoma in Situ of the Urinary Bladder: A Retrospective Multicentre Study. Bladder Cancer 2018, 4, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Kamat, A.M.; Sylvester, R.J.; Böhle, A.; Palou, J.; Lamm, D.L.; Brausi, M.; Soloway, M.; Persad, R.; Buckley, R.; Colombel, M.; et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations from the International Bladder Cancer Group. J. Clin. Oncol. 2016, 34, 1935–1944. [Google Scholar] [CrossRef]
- Balar, A.V.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.; Mourey, L.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Kamat, A.M.; Grivas, P.; et al. Keynote 057: Phase II trial of Pembrolizumab (pembro) for patients (pts) with high-risk (HR) nonmuscle invasive bladder cancer (NMIBC) unresponsive to bacillus calmette-guérin (BCG). J. Clin. Oncol. 2019, 37, 350. [Google Scholar] [CrossRef]
- FDA. Approves Pembrolizumab for BCG-Unresponsive, High-Risk Non-Muscle Invasive Bladder Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer (accessed on 6 September 2020).
- Wallis, C.; Shore, N.; Boorjian, S.; Dinney, C. Results from the phase III study of Nadofaregene Firadenovec: Safety and Efficacy in Patients with High-grade, BCG-unresponsive Non-Muscle Invasive Bladder Cancer. In Proceedings of the EAU 2020, Virtual Congress, 17–21 July 2020; Available online: https://www.urotoday.com/conference-highlights/eau-2020/bladder-cancer/123164-eau-2020-results-from-the-phase-iii-study-of-nadofaregene-firadenovec-safety-and-efficacy-in-patients-with-high-grade-bcg-unresponsive-non-muscle-invasive-bladder-cancer.html (accessed on 26 August 2020).
- Steinberg, R.L.; Thomas, L.J.; Brooks, N.; Mott, S.L.; Vitale, A.; Crump, T.; Rao, M.Y.; Daniels, M.J.; Wang, J.; Nagaraju, S.; et al. Multi-Institution Evaluation of Sequential Gemcitabine and Docetaxel as Rescue Therapy for Nonmuscle Invasive Bladder Cancer. J. Urol. 2020, 203, 902–909. [Google Scholar] [CrossRef]
- Bacillus Calmette-Guérin-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bacillus-calmette-guerin-unresponsive-nonmuscle-invasive-bladder-cancer-developing-drugs-and (accessed on 6 September 2020).
- May, M.; Helke, C.; Nitzke, T.; Vogler, H.; Hoschke, B. Survival rates after radical cystectomy according to tumor stage of bladder carcinoma at first presentation. Urol. Int. 2004, 72, 103–111. [Google Scholar] [CrossRef]
- Huguet, J.; Gaya, J.M.; Sabaté, S.; Palou, J.; Villavicencio, H. Radical cystectomy in patients with non-muscle invasive bladder cancer who fail BCG therapy. Actas Urol. Esp. 2010, 34, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Sri, D.; Lee, H.J.; El-Gemmal, S.; Backhouse, C.; Tay, A.; John, B.; Perry, M.J.; Ayres, B.E.; Issa, R. Cystectomy outcomes in patients who have failed Radiofrequency-induced Thermo-chemotherapeutic Effect Mitomycin-C (RITE-MMC) treatment for high-risk non-muscle invasive bladder cancer (HRNMIBC)-Does it complicate surgery and adversely impact oncological outcome? Urol. Oncol. 2020. [Google Scholar] [CrossRef]
- Brausi, M.; Oddens, J.; Sylvester, R.; Bono, A.; van de Beek, C.; van Andel, G.; Gontero, P.; Turkeri, L.; Marreaud, S.; Collette, S.; et al. Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: Results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur. Urol. 2014, 65, 69–76. [Google Scholar] [CrossRef]
- De Groot, A.C.; van der Meijden, A.P.M.; Conemans, J.M.H.; Maibach, H.I. Frequency and nature of cutaneous reactions to intravesical instillation of mitomycin for superficial bladder cancer. Urology 1992, 40, 16–19. [Google Scholar] [CrossRef]
- Datum van Overlijden van Personen die Ingeschreven Staan in de GBA. Available online: https://www.cbs.nl/ (accessed on 10 December 2019).
- Levensverwachting; Geslacht, Geboortegeneratie. Available online: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/80333ned/table?ts=1580821457986 (accessed on 26 August 2020).
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/ (accessed on 6 September 2020).


| Description | Subgroup | Title | ≥6 Sessions | All Patients |
|---|---|---|---|---|
| Total, n | 274 | 299 | ||
| Sex, n (%) | Female | 60 (21.9) | 65 (21.7) | |
| Male | 214 (78.1) | 234 (87.3) | ||
| Age, median (IQR) | 66 (60–74) | 67 (60–74) | ||
| Baseline histology, n (%) | CIS | 128 (46.7) | 146 (48.8) | |
| CIS + Ta | 49 (17.9) | 57 (19.1) | ||
| CIS + T1 | 23 (8.4) | 24 (8.0) | ||
| CIS only | 56 (20.4) | 65 (21.7) | ||
| Papillary | 146 (53.3) | 157 (52.5) | ||
| Ta LG 1 | 23 (8.4) | 23 (7.7) | ||
| Ta HG 2 | 105 (38.3) | 114 (38.1) | ||
| T1 HG | 18 (6.6) | 20 (6.6) | ||
| Recurrence frequency, n (%) | ≥1/year | 188 (69.1) | 202 (68.2) | |
| <1/year | 84 (30.9) | 94 (31.8) | ||
| Risk stratification (EAU), n (%) | Intermediate | 77 (28.1) | 81 (27.1) | |
| High | 197 (71.9) | 218 (72.9) | ||
| Previous BCG treatment, n (%) | Yes | 234 (85.4) | 255 (85.3) | |
| Refractory | 178 (65.0) | 194 (64.9) | ||
| Intolerant | 21 (7.7) | 23 (7.7) | ||
| No | 35 (12.8) | 39 (13.0) | ||
| Chemotherapeutic agent, n (%) | MMC | 239 (87.2) | 262 (87.6) | |
| Epirubicin | 35 (12.8) | 37 (12.4) | ||
| Dose, n (%) | Ablative | 120 (43.8) | 133 (44.5) | |
| Adjuvant | 154 (56.2) | 166 (55.5) |
| Variable | Subgroup | % 6-Month CR 3 | Unadjusted OR 4 (95% CI) | p Value | Adjusted OR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Overall (n = 137) | CIS and papillary patients with tumor at baseline | 55.5 | ||||
| Baseline histology | (Concomitant) CIS (n = 116) | 56.0 | 0.86 (0.34–2.19) | 0.76 | 0.35 (0.10–1.21) | 0.10 |
| Papillary (n = 21) | 52.4 | |||||
| Dose | Adjuvant (n = 46) | 45.7 | 0.55 (0.27–1.12) | 0.10 | 0.49 (0.23–1.08) | 0.08 |
| Ablative (n = 91) | 60.4 |
| Variable | Subgroup | % 1-Year RFS 5 (95% CI) | % 2-Year RFS (95% CI) | % 5-Year RFS (95% CI) |
|---|---|---|---|---|
| Overall (n = 204) | 78.6 (72.9–84.3) | 60.3 (53.2–67.4) | 38.1 (30.5–45.7) | |
| Baseline histology | (concomitant) CIS (n = 70) | 79.7 (69.7–89.7) | 66.5 (54.3–78.7) | 40.3 (25.2–55.4) |
| Papillary (n = 134) | 77.9 (70.8–85.0) | 57.5 (48.9–66.1) | 37.2 (28.4–46.0) | |
| Dose | Ablative (n = 73) | 86.9 (78.9–94.9) | 71.9 (60.7–83.1) | 47.6 (33.3–61.9) |
| Adjuvant (n = 131) | 74.0 (66.4–81.6) | 54.2 (45.4–63.0) | 33.9 (25.1–42.7) |
| Variables | Unadjusted HR 6 (95% CI) | p Value | Adjusted HR (95% CI) | p Value |
|---|---|---|---|---|
| Ablative vs. adjuvant dose | 0.64 (0.42–0.98) | 0.04 | 0.54 (0.34–0.85) | 0.01 |
| MMC vs. epirubicin | 1.12 (0.65–1.93) | 0.69 | 1.23 (0.71–2.14) | 0.46 |
| (Concomitant) CIS vs. papillary | 0.85 (0.56–1.30) | 0.46 | 0.94 (0.57–1.55) | 0.81 |
| Previous BCG vs. BCG naïve | 1.91 (1.04–3.50) | 0.04 | 2.07 (1.05–4.08) | 0.04 |
| Previous high vs. low tumor grade | 1.15 (0.76–1.74) | 0.51 | 1.25 (0.79–1.98) | 0.34 |
| Previous recurrence rate ≥1 vs. <1/year | 1.64 (1.06–2.52) | 0.03 | 1.39 (0.85–2.28) | 0.19 |
| Survival (n = 274) | 5-Year, % (95% CI) | 10-Year, % (95% CI) |
|---|---|---|
| OS 7 | 72.3 (66.4–87.2) | 51.0 (43.4–58.6) |
| RS 8 | 80.6 (74.0–87.1) | 65.1 (55.2–75.1) |
| CSS 7 | 86.6 (81.7–91.5) | 77.6 (70.3–84.9) |
| Adverse Event (n = 294) | Any Grade 9, n (%) | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) |
|---|---|---|---|---|
| Spasms | 183 (62.2) | 85 (28.9) | 93 (31.6) | 5 (1.7) |
| Pain | 82 (27.1) | 60 (20.1) | 17 (5.7) | 5 (1.7) |
| Catheter problems | 52 (17.7) | 30 (10.2) | 18 (6.1) | 4 (1.4) |
| Dysuria | 156 (53.1) | 126 (42.9) | 26 (8.8) | 4 (1.4) |
| Hematuria | 88 (29.9) | 83 (28.2) | 5 (1.7) | 0 (0) |
| Urinary tract infection | 46 (15.6) | 0 (0) | 39 (13.3) | 7 (2.4) |
| Nocturia | 43 (14.6) | 22 (7.5) | 16 (5.4) | 5 (1.7) |
| Incontinence | 18 (6.1) | 12 (4.1) | 6 (2.0) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brummelhuis, I.S.G.; Wimper, Y.; Witjes-van Os, H.G.J.M.; Arends, T.J.H.; van der Heijden, A.G.; Witjes, J.A. Long-Term Experience with Radiofrequency-Induced Hyperthermia Combined with Intravesical Chemotherapy for Non-Muscle Invasive Bladder Cancer. Cancers 2021, 13, 377. https://doi.org/10.3390/cancers13030377
Brummelhuis ISG, Wimper Y, Witjes-van Os HGJM, Arends TJH, van der Heijden AG, Witjes JA. Long-Term Experience with Radiofrequency-Induced Hyperthermia Combined with Intravesical Chemotherapy for Non-Muscle Invasive Bladder Cancer. Cancers. 2021; 13(3):377. https://doi.org/10.3390/cancers13030377
Chicago/Turabian StyleBrummelhuis, Iris S. G., Yvonne Wimper, Hilde G. J. M. Witjes-van Os, Tom J. H. Arends, Antoine G. van der Heijden, and J. Alfred Witjes. 2021. "Long-Term Experience with Radiofrequency-Induced Hyperthermia Combined with Intravesical Chemotherapy for Non-Muscle Invasive Bladder Cancer" Cancers 13, no. 3: 377. https://doi.org/10.3390/cancers13030377
APA StyleBrummelhuis, I. S. G., Wimper, Y., Witjes-van Os, H. G. J. M., Arends, T. J. H., van der Heijden, A. G., & Witjes, J. A. (2021). Long-Term Experience with Radiofrequency-Induced Hyperthermia Combined with Intravesical Chemotherapy for Non-Muscle Invasive Bladder Cancer. Cancers, 13(3), 377. https://doi.org/10.3390/cancers13030377