HIF2alpha-Associated Pseudohypoxia Promotes Radioresistance in Pheochromocytoma: Insights from 3D Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
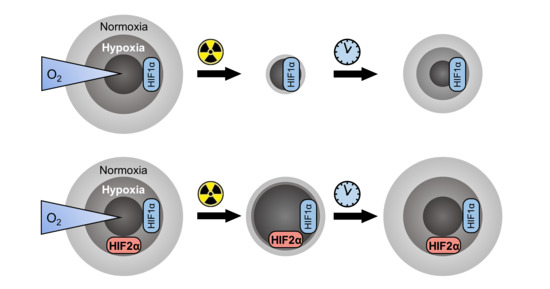
2.1. Response of MPCwt Spheroids to External X-ray Irradiation
2.2. Effect of Hif2α Expression on Response of MPC Spheroids to External X-ray Irradiation
2.3. Impact of G418 and DMSO on the Experimental Outcome of Spheroid Irradiation Treatment
2.4. Effects of Hif2α Expression on the Response of MPC Spheroids to Incubation with [177Lu]LuCl3
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Routine Cultivation
4.2. Microscopy
4.3. External Beam Radiation Treatment with X-ray
4.4. Radionuclide Treatment with [177Lu]LuCl3
4.5. Statistical Analyses
4.6. Analyses of Treatment Effects on Spheroids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMSO | dimethylsulfoxide |
| DOTA | 1,4,7,10 tetraazacyclododecane-N,N′,N″,N″′ tetraacetic acid |
| G418 | geneticin |
| GAD | growth arrest dose |
| HIF | hypoxia-inducible factor |
| MPC | mouse pheochromocytoma cell line |
| PCCs/PGLs | pheochromocytomas and paragangliomas |
| SCD50 | half-maximal spheroid control dose |
| %SCP | spheroid control probability |
| %SG | relative spheroid growth |
| SSTR2 | somatostatin type 2 receptor |
| TATE | (Tyr3)octreotate |
References
- Harari, A.; Inabnet, W.B. Malignant pheochromocytoma: A review. Am. J. Surg. 2011, 201, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Buzzoni, R.; Pusceddu, S.; Damato, A.; Meroni, E.; Aktolun, C.; Milione, M.; Mazzaferro, V.; De Braud, F.; Spreafico, C.; Maccauro, M.; et al. Malignant pheochromocytoma and paraganglioma: Future considerations for therapy. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 153–160. [Google Scholar] [PubMed]
- Klöppel, G. Tumoren des Nebennierenmarks und der Paraganglien. Pathologe 2003, 24, 280–286. [Google Scholar] [CrossRef]
- Salmenkivi, K.; Heikkilä, P.; Haglund, C.; Arola, J. Malignancy in pheochromocytomas. Review article. APMIS 2004, 112, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Bornstein, S.R.; Brouwers, F.M.; Cheung, N.-K.V.; Dahia, P.L.; De Krijger, R.R.; Giordano, T.J.; Greene, L.A.; Goldstein, D.S.; Lehnert, H.; et al. Malignant pheochromocytoma: Current status and initiatives for future progress. Endocr. Relat. Cancer 2004, 11, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Brogsitter, C.; Pinkert, J.; Bredow, J.; Kittner, T.; Kotzerke, J. Enhanced tumor uptake in neuroendocrine tu-mors after intraarterial application of 131I-MIBG. J. Nucl. Med. 2005, 46, 2112–2116. [Google Scholar] [PubMed]
- Mak, I.Y.F.; Hayes, A.R.; Khoo, B.; Grossman, A. Peptide receptor radionuclide therapy as a novel treatment for metastatic and invasive phaeochromocytoma and paraganglioma. Neuroendocrinology 2019, 109, 287–298. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, M.R.A.; Knapp, F.F. Lutetium-177 Therapeutic radiopharmaceuticals: Linking chemistry, radiochemistry, and practical applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Pavel, M.E.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [Green Version]
- Zovato, S.; Kumanova, A.; Demattè, S.; Sansovini, M.; Bodei, L.; Di Sarra, D.; Casagranda, E.; Severi, S.; Ambrosetti, A.; Schiavi, F.; et al. Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL). Horm. Metab. Res. 2012, 44, 411–414. [Google Scholar] [CrossRef]
- Zandee, W.T.; Feelders, R.A.; Duijzentkunst, D.A.S.; Hofland, J.; Metselaar, R.M.; Oldenburg, R.A.; van Linge, A.; Kam, B.L.; Teunissen, J.J.M.; Korpershoek, E.; et al. Treatment of inoperable or metastatic paragangliomas and pheochromocytomas with peptide receptor radionuclide therapy using 177Lu-DOTATATE. Eur. J. Endocrinol. 2019, 181, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, D.; Doddamane, I.; Cheng, D. Targeted radionuclide therapy. Cancers 2011, 3, 3838–3855. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Shilyagina, N.Y.; Vodeneev, V.; Zvyagin, A.V. Targeted radionuclide therapy of human tumors. Int. J. Mol. Sci. 2015, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, M.; Bergmann, R.; Peitzsch, M.; Zenker, E.F.; Cartellieri, M.; Bachmann, M.; Ehrhart-Bornstein, M.; Block, N.L.; Schally, A.V.; Eisenhofer, G.; et al. Multimodal somatostatin receptor theranostics using [64Cu]Cu-/[177Lu]Lu-DOTA-(Tyr3)) octreotate and AN-238 in a Mouse Pheochromocytoma Model. Theranostics 2016, 6, 650–665. [Google Scholar] [CrossRef]
- Wolf, K.I.; Jha, A.; van Berkel, A.; Wild, D.; Janssen, I.; Millo, C.M.; Janssen, M.J.R.; Gonzales, M.K.; Timmers, H.J.K.M.; Pacak, K. Eruption of metastatic paraganglioma after successful therapy with 177Lu/90Y-DOTATOC and 177Lu-DOTATATE. Nucl. Med. Mol. Imaging 2019, 53, 223–230. [Google Scholar] [CrossRef]
- Teunissen, J.J.M.; Kwekkeboom, D.J.; Valkema, R.; Krenning, E.P. Nuclear medicine techniques for the imaging and treatment of neuroendocrine tumours. Endocr. Relat. Cancer 2011, 18, S27–S51. [Google Scholar] [CrossRef]
- Kong, G.; Grozinsky-Glasberg, S.; Hofman, M.S.; Callahan, J.; Meirovitz, A.; Maimon, O.; Pattison, D.A.; Gross, D.J.; Hicks, R.J. Efficacy of peptide receptor radionuclide therapy for functional metastatic paraganglioma and pheochromocytoma. J. Clin. Endocrinol. Metab. 2017, 102, 3278–3287. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.P.; Ballal, S.; Bal, C. Concomitant 177Lu-DOTATATE and capecitabine therapy in malignant paragangliomas. EJNMMI Res. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Bertout, J.A.; Patel, S.A.; Simon, M.C. The impact of O2 availability on human cancer. Nat. Rev. Cancer 2008, 8, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Du, F.; Shen, G.; Zheng, F.; Xu, B. The role of hypoxia-inducible factor-2 in digestive system cancers. Cell Death Dis. 2015, 6, e1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jochmanová, I.; Yang, C.; Zhuang, Z.; Pacak, K. Hypoxia-inducible factor signaling in pheochromocytoma: Turning the rudder in the right direction. J. Natl. Cancer Inst. 2013, 105, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Keith, B.; Johnson, R.S.; Simon, M.C. HIF1α and HIF2α: Sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 2011, 12, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Wigerup, C.; Påhlman, S.; Bexell, D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol. Ther. 2016, 164, 152–169. [Google Scholar] [CrossRef] [Green Version]
- Taïeb, D.; Pacak, K. New Insights into the nuclear imaging phenotypes of Cluster 1 pheochromocytoma and paraganglioma. Trends Endocrinol. Metab. 2017, 28, 807–817. [Google Scholar] [CrossRef]
- Seifert, V.; Liers, J.; Kniess, T.; Richter, S.; Bechmann, N.; Feldmann, A.; Bachmann, M.; Eisenhofer, G.; Pietzsch, J.; Ullrich, M. Fluorescent mouse pheochromocytoma spheroids expressing hypoxia-inducible factor 2 alpha: Morphologic and radiopharmacologic characterization. J. Cell. Biotechnol. 2019, 5, 135–151. [Google Scholar] [CrossRef]
- Bechmann, N.; Poser, I.; Seifert, V.; Greunke, C.; Ullrich, M.; Qin, N.; Walch, A.; Peitzsch, M.; Robledo, M.; Pacak, K.; et al. Impact of extrinsic and intrinsic hypoxia on catecholamine biosynthesis in absence or presence of Hif2α in pheochromocytoma cells. Cancers 2019, 11, 594. [Google Scholar] [CrossRef] [Green Version]
- Burnichon, N.; Vescovo, L.; Amar, L.; Libé, R.; de Reynies, A.; Venisse, A.; Jouanno, E.; Laurendeau, I.; Parfait, B.; Bertherat, J.; et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum. Mol. Genet. 2011, 20, 3974–3985. [Google Scholar] [CrossRef]
- López-Jiménez, E.; Gómez-López, G.; Leandro-García, L.J.; Muñoz, I.; Schiavi, F.; Montero-Conde, C.; de Cubas, A.A.; Ramires, R.; Landa, I.; Leskelä, S.; et al. Research Resource: Transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol. Endocrinol. 2010, 24, 2382–2391. [Google Scholar] [CrossRef] [Green Version]
- Bechmann, N.; Moskopp, M.L.; Ullrich, M.; Calsina, B.; Wallace, P.W.; Richter, S.; Friedemann, M.; Langton, K.; Fliedner, S.M.J.; Timmers, H.J.; et al. HIF2α supports pro-metastatic behavior in pheochromocytomas/paragangliomas. Endocr. Relat. Cancer 2020, 27, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Gholami, Y.H.; Willowson, K.P.; Forwood, N.J.; Harvie, R.; Hardcastle, N.; Bromley, R.; Ryu, H.; Yuen, S.; Howell, V.M.; Kuncic, Z.; et al. Comparison of radiobiological parameters for 90Y radionuclide therapy (RNT) and external beam radiotherapy (EBRT) in vitro. EJNMMI Phys. 2018, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, R.; Andreeff, M.; Oehme, L.; Kotzerke, J. Dosimetry of cell-monolayers in multiwell plat. Nuklearmedizin 2009, 48, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Marcatili, S.; Pichard, A.; Courteau, A.; Ladjohounlou, R.; Navarro-Teulon, I.; Repetto-Llamazares, A.; Heyerdahl, H.; Dahle, J.; Pouget, J.P.; Bardiès, M. Realistic multi-cellular dosimetry for177Lu-labelled antibodies: Model and application. Phys. Med. Biol. 2016, 61, 6935–6952. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Doctor, A.; Seifert, V.; Ullrich, M.; Hauser, S.; Pietzsch, J. Three-dimensional cell culture systems in radiopharmaceutical cancer research. Cancers 2020, 12, 2765. [Google Scholar] [CrossRef]
- Mueller-Klieser, W. Multicellular spheroids. J. Cancer Res. Clin. Oncol. 1987, 113, 101–122. [Google Scholar] [CrossRef]
- Kempf, H.; Bleicher, M.; Meyer-Hermann, M. Spatio-temporal cell dynamics in tumour spheroid irradiation. Eur. Phys. J. D 2010, 60, 177–193. [Google Scholar] [CrossRef]
- Dale, R.; Carabe-Fernandez, A. The radiobiology of conventional radiotherapy and its application to radionuclide therapy. Cancer Biother. Radiopharm. 2005, 20, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Folkman, J.; Hochberg, M. Self-regulation of growth in three dimensions. J. Exp. Med. 1973, 138, 745–753. [Google Scholar] [CrossRef]
- Qin, N.; de Cubas, A.A.; Garcia-Martin, R.; Richter, S.; Peitzsch, M.; Menschikowski, M.; Lenders, J.W.; Timmers, H.J.; Mannelli, M.; Opocher, G.; et al. Opposing effects of HIF1α and HIF2α on chromaffin cell phenotypic features and tumor cell proliferation: Insights from MYC-associated factor X. Int. J. Cancer 2014, 135, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T.; et al. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell 2017, 31, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Dahia, P.L.M. Pheochromocytoma and paraganglioma pathogenesis: Learning from genetic heterogeneity. Nat. Rev. Cancer 2014, 14, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Rossignol, F.; Matthay, M.A.; Mounier, R.; Couette, S.; Clottes, E.; Clerici, C. Prolonged Hypoxia differentially regulates hypoxia-inducible factor (HIF)-1α and HIF-2α expression in lung epithelial cells. J. Biol. Chem. 2004, 279, 14871–14878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhikrivetskaya, S.O.; Snezhkina, A.V.; Zaretsky, A.R.; Alekseev, B.Y.; Pokrovsky, A.V.; Golovyuk, A.L.; Melnikova, N.V.; Stepanov, O.A.; Kalinin, D.V.; Moskalev, A.A.; et al. Molecular markers of paragangliomas/pheochromocytomas. Oncotarget 2017, 8, 25756–25782. [Google Scholar] [CrossRef] [Green Version]
- Favier, J.; Igaz, P.; Burnichon, N.; Amar, L.; Libé, R.; Badoual, C.; Tissier, F.; Bertherat, J.; Plouin, P.-F.; Jeunemaitre, X.; et al. Rationale for Anti-angiogenic therapy in pheochromocytoma and paraganglioma. Endocr. Pathol. 2011, 23, 34–42. [Google Scholar] [CrossRef]
- Martínez-Salgado, C.; Lopez-Hernandez, F.J.; López-Novoa, J.M. Glomerular nephrotoxicity of aminoglycosides. Toxicol. Appl. Pharmacol. 2007, 223, 86–98. [Google Scholar] [CrossRef]
- Mingeot-Leclercq, M.-P.; Tulkens, P.M. Aminoglycosides: Nephrotoxicity. Antimicrob. Agents Chemother. 1999, 43, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Därr, R.; Nambuba, J.; Del Rivero, J.; Janssen, I.; Merino, M.; Todorovic, M.; Balint, B.; Jochmanova, I.; Prchal, J.T.; Lechan, R.M.; et al. Novel insights into the polycythemia–paraganglioma–somatostatinoma syndrome. Endocr. Relat. Cancer 2016, 23, 899–908. [Google Scholar] [CrossRef] [Green Version]
- Perez, C.A.; Grigsby, P.W.; Chao, K.S.; Mutch, D.G.; Lockett, M.A. Tumor size, irradiation dose, and long-term outcome of carcinoma of uterine cervix. Int. J. Radiat. Oncol. 1998, 41, 307–317. [Google Scholar] [CrossRef]
- Gaze, M.N.; Mairs, R.J.; Boyack, S.M.; Wheldon, T.E.; Barrett, A. 131I-meta-iodobenzylguanidine therapy in neuroblastoma spheroids of different sizes. Br. J. Cancer 1992, 66, 1048–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingargiola, M.; Runge, R.; Heldt, J.-M.; Freudenberg, R.; Steinbach, J.; Cordes, N.; Baumann, M.; Kotzerke, J.; Brockhoff, G.; Kunz-Schughart, L.A. Potential of a Cetuximab-based radioimmunotherapy combined with external irradiation manifests in a 3-D cell assay. Int. J. Cancer 2014, 135, 968–980. [Google Scholar] [CrossRef] [Green Version]
- Budach, W.; Budach, V.; Stuschke, M.; Dinges, S.; Sack, H. The TCD50 and regrowth delay assay in human tumor xenografts: Differences and implications. Int. J. Radiat. Oncol. 1993, 25, 259–268. [Google Scholar] [CrossRef]
- Ullrich, M.; Bergmann, R.; Peitzsch, M.; Cartellieri, M.; Qin, N.; Ehrhart-Bornstein, M.; Block, N.L.; Schally, A.V.; Pietzsch, J.; Eisenhofer, G.; et al. In vivo fluorescence imaging and urinary monoamines as surrogate biomarkers of disease progression in a mouse model of pheochromocytoma. Endocrinology 2014, 155, 4149–4156. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. Mechanisms of cell killing response from low linear energy transfer (LET) radiation originating from 177Lu radioimmunotherapy targeting disseminated intraperitoneal tumor xenografts. Int. J. Mol. Sci. 2016, 17, 736. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Radiation Treatment | Diameter Changes after Treatment Start [µm/day] | ||||
|---|---|---|---|---|---|
| MPCwt | MPC +HIF2α | MPC +EV | MPC +HIF2α | ||
| External X-ray irradiation | |||||
| X-ray dose [Gy] | 0–3 days | 0–3 days | 0–3 days | 3–6 days | |
| 0 | 47 ± 4 | 67 ± 2 | 49 ± 3 | 75 ± 2 | |
| 4 | −43 ± 2 | 34 ± 2 | −24 ± 2 | 48 ± 2 | |
| 8 | −75 ± 1 | 19 ± 2 | −39 ± 2 | 19 ± 1 | |
| 12 | −87 ± 2 | 16 ± 2 | −54 ± 2 | −3.4 ± 2 | |
| 16 | −89 ± 1 | 18 ± 2 | −57 ± 1 | −10 ± 2 | |
| 20 | −102 ± 2 | 11 ± 3 | −52 ± 1 | −22 ± 2 | |
| 25 | −108 ± 1 | 8.3 ± 3 | −60 ± 2 | −21 ± 2 | |
| 40 | −118 ± 1 | 1.7 ± 3 | −66 ± 4 | −26 ± 4 | |
| rp | −0.84 | −0.84 | −0.72 | −0.80 | |
| p | 0.01 | 0.01 | 0.05 | 0.05 | |
| Incubation with [177Lu]LuCl3 | |||||
| Initial AV [MBq/mL] | (approx. β− dose [Gy]) | 0–3 days | 0–3 days | 0–3 days | 3–6 days |
| 0 | (0) | n. a. | 69 ± 2 | 52 ± 2 | 60 ± 3 |
| 0.03 | (1) | n. a. | 64 ± 2 | 24 ± 3 | 47 ± 4 |
| 0.05 | (2) | n. a. | 58 ± 2 | −1.5 ± 3 | 20 ± 4 |
| 0.10 | (4) | n. a. | 49 ± 4 | −6.7 ± 2 | 0.8 ± 6 |
| 0.15 | (6) | n. a. | 44 ± 5 | −18 ± 2 | −13 ± 13 |
| 0.25 | (10) | n. a. | 31 ± 2 | −23 ± 1 | −40 ± 5 |
| rp | n. a. | −0.97 | −0.85 | −0.99 | |
| p | n. a. | 0.01 | 0.05 | 0.001 | |
| Treatment | Parameter | Measurand [Unit] | MPC + EV | MPC +HIF2α | p |
|---|---|---|---|---|---|
| X-ray | GAD | X-ray dose [Gy] | 5.0 ± 0.3 | 16 ± 1.0 | 0.001 |
| [177]LuCl3 | GAD | Initial AV [MBq/mL] | 0.3 ± 0.02 | 1.7 ± 0.7 | 0.05 |
| [177]LuCl3 | GAD | (approx. β− dose [Gy]) | (2.2 ± 0.2) | (14 ± 6.0) | |
| X-ray | SCD50 | X-ray dose [Gy] | 17 ± 0.2 | 21 ± 0.3 | 0.001 |
| [177]LuCl3 | SCD50 | Initial AV [MBq/mL] | 0.3 ± 0.02 | 0.6 ± 0.02 | 0.001 |
| [177]LuCl3 | SCD50 | (approx. β− dose [Gy]) | (2.7 ± 0.1) | (4.6 ± 0.2) |
| Treatment | MPCwt | MPC + EV | MPC +HIF2α | |||
|---|---|---|---|---|---|---|
| n | d [µm] | n | d [µm] | n | d [µm] | |
| X-ray | 10 | 616 ± 2 | 10 | 479 ± 3 | 10 | 499 ± 3 |
| [177Lu]LuCl3 | n. a. | n. a. | 20 † | 448 ± 3 | 20 † | 492 ± 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seifert, V.; Richter, S.; Bechmann, N.; Bachmann, M.; Ziegler, C.G.; Pietzsch, J.; Ullrich, M. HIF2alpha-Associated Pseudohypoxia Promotes Radioresistance in Pheochromocytoma: Insights from 3D Models. Cancers 2021, 13, 385. https://doi.org/10.3390/cancers13030385
Seifert V, Richter S, Bechmann N, Bachmann M, Ziegler CG, Pietzsch J, Ullrich M. HIF2alpha-Associated Pseudohypoxia Promotes Radioresistance in Pheochromocytoma: Insights from 3D Models. Cancers. 2021; 13(3):385. https://doi.org/10.3390/cancers13030385
Chicago/Turabian StyleSeifert, Verena, Susan Richter, Nicole Bechmann, Michael Bachmann, Christian G. Ziegler, Jens Pietzsch, and Martin Ullrich. 2021. "HIF2alpha-Associated Pseudohypoxia Promotes Radioresistance in Pheochromocytoma: Insights from 3D Models" Cancers 13, no. 3: 385. https://doi.org/10.3390/cancers13030385
APA StyleSeifert, V., Richter, S., Bechmann, N., Bachmann, M., Ziegler, C. G., Pietzsch, J., & Ullrich, M. (2021). HIF2alpha-Associated Pseudohypoxia Promotes Radioresistance in Pheochromocytoma: Insights from 3D Models. Cancers, 13(3), 385. https://doi.org/10.3390/cancers13030385