Experimental Models for Studying HPV-Positive and HPV-Negative Penile Cancer: New Tools for An Old Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Role of HPV in Penile Cancer
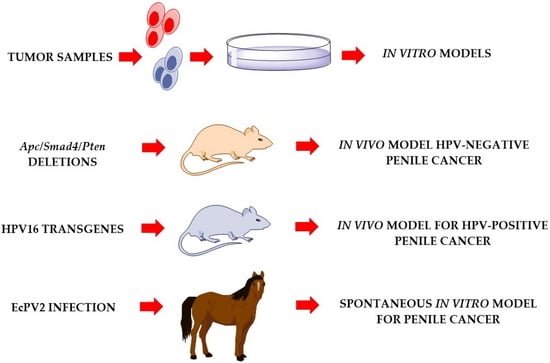
3. Cell-Based Models of Penile Cancer
4. In Vivo Animal Models
4.1. Mouse Penis: Anatomy and Histology
4.2. HPV-Negative Penile Cancer in SMAD4/APC Double Knockout Mice
4.3. In Vivo Models for HPV-Positive Penile Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Douglawi, A.; Masterson, T.A. Updates on the epidemiology and risk factors for penile cancer. Transl. Androl. Urol. 2017, 6, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, R.W.P.; Pinho, J.D.; Moreno, J.S.; Garbis, D.; do Nascimento, A.M.T.; Larges, J.S.; Calixto, J.R.R.; Ramalho, L.N.Z.; da Silva, A.A.M.; Nogueira, L.R.; et al. Penile cancer in Maranhao, Northeast Brazil: The highest incidence globally? BMC Urol. 2018, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=1&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=1&include_nmsc_other=1 (accessed on 3 January 2021).
- Alemany, L.; Cubilla, A.; Halec, G.; Kasamatsu, E.; Quiros, B.; Masferrer, E.; Tous, S.; Lloveras, B.; Hernandez-Suarez, G.; Lonsdale, R.; et al. Role of Human Papillomavirus in Penile Carcinomas Worldwide. Eur. Urol. 2016, 69, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Olesen, T.B.; Sand, F.L.; Rasmussen, C.L.; Albieri, V.; Toft, B.G.; Norrild, B.; Munk, C.; Kjaer, S.K. Prevalence of human papillomavirus DNA and p16(INK4a) in penile cancer and penile intraepithelial neoplasia: A systematic review and meta-analysis. Lancet Oncol. 2019, 20, 145–158. [Google Scholar] [CrossRef]
- Pow-Sang, M.R.; Benavente, V.; Pow-Sang, J.E.; Morante, C.; Meza, L.; Baker, M.; Pow-Sang, J.M. Cancer of the penis. Cancer Control 2002, 9, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Pompeo, A.; Heyns, C.; Abrams, P. Penile Cancer; Société Internationale d’Urologie (SIU): Montréal, QC, Canada, 2009. [Google Scholar]
- Dillner, J.; von Krogh, G.; Horenblas, S.; Meijer, C.J. Etiology of squamous cell carcinoma of the penis. Scand. J. Urol. Nephrol. Suppl. 2000, 189–193. [Google Scholar] [CrossRef]
- Hakenberg, O.W.; Comperat, E.M.; Minhas, S.; Necchi, A.; Protzel, C.; Watkin, N. EAU guidelines on penile cancer: 2014 Update. Eur. Urol. 2015, 67, 142–150. [Google Scholar] [CrossRef]
- Clark, P.E.; Spiess, P.E.; Agarwal, N.; Biagioli, M.C.; Eisenberger, M.A.; Greenberg, R.E.; Herr, H.W.; Inman, B.A.; Kuban, D.A.; Kuzel, T.M.; et al. Penile cancer: Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2013, 11, 594–615. [Google Scholar] [CrossRef]
- Marieb, E.; Wilhelm, P.B.; Mallatt, J. Human Anatomy, 7th ed.; Pearson Education, Inc.: London, UK, 2014. [Google Scholar]
- Hellberg, D.; Valentin, J.; Eklund, T.; Nilsson, S. Penile cancer: Is there an epidemiological role for smoking and sexual behaviour? Br. Med. J. (Clin. Res. Ed.) 1987, 295, 1306–1308. [Google Scholar] [CrossRef] [Green Version]
- Marchionne, E.; Perez, C.; Hui, A.; Khachemoune, A. Penile squamous cell carcinoma: A review of the literature and case report treated with Mohs micrographic surgery. An. Bras. Dermatol. 2017, 92, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Harish, K.; Ravi, R. The role of tobacco in penile carcinoma. Br. J. Urol. 1995, 75, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Cubilla, A.L.; Velazquez, E.F.; Amin, M.B.; Epstein, J.; Berney, D.M.; Corbishley, C.M.; Members of the ISUP Penile Tumor Panel. The World Health Organisation 2016 classification of penile carcinomas: A review and update from the International Society of Urological Pathology expert-driven recommendations. Histopathology 2018, 72, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Maggi-Galluzzi, C.; Cubilla, A.L. Tumors of the Prostate Gland, Seminal Vesicles, Penis, and Scrotum; American Registry of Pathology and AFIP: Washington, DC, USA, 2020. [Google Scholar]
- Gregoire, L.; Cubilla, A.L.; Reuter, V.E.; Haas, G.P.; Lancaster, W.D. Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J. Natl. Cancer Inst. 1995, 87, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.A.; Kleter, B.; Zhou, M.; Ayala, G.; Cubilla, A.L.; Quint, W.G.V.; Pirog, E.C. Detection and Typing of Human Papillomavirus DNA in Penile Carcinoma: Evidence for Multiple Independent Pathways of Penile Carcinogenesis. Am. J. Pathol. 2001, 159, 1211–1218. [Google Scholar] [CrossRef]
- Miralles-Guri, C.; Bruni, L.; Cubilla, A.L.; Castellsague, X.; Bosch, F.X.; de Sanjose, S. Human papillomavirus prevalence and type distribution in penile carcinoma. J. Clin. Pathol. 2009, 62, 870–878. [Google Scholar] [CrossRef]
- Cubilla, A.L.; Velazquez, E.F.; Young, R.H. Pseudohyperplastic squamous cell carcinoma of the penis associated with lichen sclerosus. An extremely well-differentiated, nonverruciform neoplasm that preferentially affects the foreskin and is frequently misdiagnosed: A report of 10 cases of a distinctive clinicopathologic entity. Am. J. Surg. Pathol. 2004, 28, 895–900. [Google Scholar] [CrossRef]
- Cunha, I.W.; Guimaraes, G.C.; Soares, F.; Velazquez, E.; Torres, J.J.; Chaux, A.; Ayala, G.; Cubilla, A.L. Pseudoglandular (adenoid, acantholytic) penile squamous cell carcinoma: A clinicopathologic and outcome study of 7 patients. Am. J. Surg. Pathol. 2009, 33, 551–555. [Google Scholar] [CrossRef]
- Kraus, F.T.; Perezmesa, C. Verrucous carcinoma. Clinical and pathologic study of 105 cases involving oral cavity, larynx and genitalia. Cancer 1966, 19, 26–38. [Google Scholar] [CrossRef]
- Barreto, J.E.; Velazquez, E.F.; Ayala, E.; Torres, J.; Cubilla, A.L. Carcinoma cuniculatum: A distinctive variant of penile squamous cell carcinoma: Report of 7 cases. Am. J. Surg. Pathol. 2007, 31, 71–75. [Google Scholar] [CrossRef]
- Chaux, A.; Soares, F.; Rodriguez, I.; Barreto, J.; Lezcano, C.; Torres, J.; Velazquez, E.F.; Cubilla, A.L. Papillary squamous cell carcinoma, not otherwise specified (NOS) of the penis: Clinicopathologic features, differential diagnosis, and outcome of 35 cases. Am. J. Surg. Pathol. 2010, 34, 223–230. [Google Scholar] [CrossRef]
- Cubilla, A.L.; Ayala, M.T.; Barreto, J.E.; Bellasai, J.G.; Noel, J.C. Surface adenosquamous carcinoma of the penis. A report of three cases. Am. J. Surg. Pathol. 1996, 20, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.F.; Melamed, J.; Barreto, J.E.; Aguero, F.; Cubilla, A.L. Sarcomatoid carcinoma of the penis: A clinicopathologic study of 15 cases. Am. J. Surg. Pathol. 2005, 29, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Cubilla, A.L.; Reuter, V.E.; Gregoire, L.; Ayala, G.; Ocampos, S.; Lancaster, W.D.; Fair, W. Basaloid squamous cell carcinoma: A distinctive human papilloma virus-related penile neoplasm: A report of 20 cases. Am. J. Surg. Pathol. 1998, 22, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Cubilla, A.L.; Velazques, E.F.; Reuter, V.E.; Oliva, E.; Mihm, M.C., Jr.; Young, R.H. Warty (condylomatous) squamous cell carcinoma of the penis: A report of 11 cases and proposed classification of ‘verruciform’ penile tumors. Am. J. Surg. Pathol. 2000, 24, 505–512. [Google Scholar] [CrossRef]
- Chaux, A.; Tamboli, P.; Ayala, A.; Soares, F.; Rodriguez, I.; Barreto, J.; Cubilla, A.L. Warty-basaloid carcinoma: Clinicopathological features of a distinctive penile neoplasm. Report of 45 cases. Mod. Pathol. 2010, 23, 896–904. [Google Scholar] [CrossRef] [Green Version]
- Cubilla, A.L.; Lloveras, B.; Alemany, L.; Alejo, M.; Vidal, A.; Kasamatsu, E.; Clavero, O.; Alvarado-Cabrero, I.; Lynch, C.; Velasco-Alonso, J.; et al. Basaloid squamous cell carcinoma of the penis with papillary features: A clinicopathologic study of 12 cases. Am. J. Surg. Pathol. 2012, 36, 869–875. [Google Scholar] [CrossRef]
- Sanchez, D.F.; Rodriguez, I.M.; Piris, A.; Canete, S.; Lezcano, C.; Velazquez, E.F.; Fernandez-Nestosa, M.J.; Mendez-Pena, J.E.; Hoang, M.P.; Cubilla, A.L. Clear Cell Carcinoma of the Penis: An HPV-related Variant of Squamous Cell Carcinoma: A Report of 3 Cases. Am. J. Surg. Pathol. 2016, 40, 917–922. [Google Scholar] [CrossRef]
- Mentrikoski, M.J.; Frierson, H.F., Jr.; Stelow, E.B.; Cathro, H.P. Lymphoepithelioma-like carcinoma of the penis: Association with human papilloma virus infection. Histopathology 2014, 64, 312–315. [Google Scholar] [CrossRef]
- Canete-Portillo, S.; Clavero, O.; Sanchez, D.F.; Silvero, A.; Abed, F.; Rodriguez, I.M.; Ayala, G.; Alemany, L.; Munoz, N.; de Sanjose, S.; et al. Medullary Carcinoma of the Penis: A Distinctive HPV-related Neoplasm: A Report of 12 Cases. Am. J. Surg. Pathol. 2017, 41, 535–540. [Google Scholar] [CrossRef]
- Fetsch, J.F.; Davis, C.J., Jr.; Miettinen, M.; Sesterhenn, I.A. Leiomyosarcoma of the penis: A clinicopathologic study of 14 cases with review of the literature and discussion of the differential diagnosis. Am. J. Surg. Pathol. 2004, 28, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Chaux, A.; Reuter, V.; Lezcano, C.; Velazquez, E.; Codas, R.; Cubilla, A.L. Autopsy findings in 14 patients with penile squamous cell carcinoma. Int. J. Surg. Pathol. 2011, 19, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Ren, W.; Xue, B.; Fan, Y.; Jiang, Y.; Zeng, C.; Li, Y.; Zu, X. Prognostic factors in patients with penile cancer after surgical management. World J. Urol. 2018, 36, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.; Morse, M.J.; Herr, H.W.; Sogani, P.C.; Whitmore, W.F., Jr. Penile cancer: Relation of extent of nodal metastasis to survival. J. Urol. 1987, 137, 880–882. [Google Scholar] [CrossRef]
- Kamel, M.H.; Bissada, N.; Warford, R.; Farias, J.; Davis, R. Organ Sparing Surgery for Penile Cancer: A Systematic Review. J. Urol. 2017, 198, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, J.M.; Djajadiningrat, R.S.; van Muilekom, E.A.; Graafland, N.M.; Horenblas, S.; Aaronson, N.K. Quality of life for patients treated for penile cancer. J. Urol. 2014, 192, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, M.; Li, Y.; Wang, Y.; Wen, S.; Jun, F. Overexpression of ID1 promotes tumor progression in penile squamous cell carcinoma. Oncol. Rep. 2019, 41, 1091–1100. [Google Scholar] [CrossRef]
- Hu, X.; Chen, M.; Li, Y.; Wang, Y.; Wen, S.; Jun, F. Aberrant CEACAM19 expression is associated with metastatic phenotype in penile cancer. Cancer Manag. Res. 2019, 11, 715–725. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Chen, M.; Liu, W.; Li, Y.; Fu, J. Preoperative plasma IGFBP2 is associated with nodal metastasis in patients with penile squamous cell carcinoma. Urol. Oncol. 2019, 37, 452–461. [Google Scholar] [CrossRef]
- Mo, M.; Tong, S.; Li, T.; Zu, X.; Hu, X. Serum CXCL13 Level is Associated with Tumor Progression and Unfavorable Prognosis in Penile Cancer. OncoTargets Ther. 2020, 13, 8757–8769. [Google Scholar] [CrossRef]
- Chen, K.L.; Eberli, D.; Yoo, J.J.; Atala, A. Bioengineered corporal tissue for structural and functional restoration of the penis. Proc. Natl. Acad. Sci. USA 2010, 107, 3346–3350. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Landford, W.N.; Garza, M.; Suarez, A.; Zhou, Z.; Coon, D. Complete Human Penile Scaffold for Composite Tissue Engineering: Organ Decellularization and Characterization. Sci. Rep. 2019, 9, 16368. [Google Scholar] [CrossRef]
- Kajbafzadeh, A.M.; Abbasioun, R.; Sabetkish, N.; Sabetkish, S.; Habibi, A.A.; Tavakkolitabassi, K. In vivo human corpus cavernosum regeneration: Fabrication of tissue-engineered corpus cavernosum in rat using the body as a natural bioreactor. Int. Urol. Nephrol. 2017, 49, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Du, X.; Li, K.; Chen, Y.; Guan, Y.; Zhao, X.; Niu, G.; Luan, Y.; Zhang, D.; Sun, C.; et al. Construction of engineered corpus cavernosum with primary mesenchymal stem cells in vitro. Sci. Rep. 2017, 7, 18053. [Google Scholar] [CrossRef] [PubMed]
- Buonerba, C.; Di Lorenzo, G.; Pond, G.; Carteni, G.; Scagliarini, S.; Rozzi, A.; Quevedo, F.J.; Dorff, T.; Nappi, L.; Lanzetta, G.; et al. Prognostic and Predictive Factors in Patients with Advanced Penile Cancer Receiving Salvage (2nd or Later Line) Systemic Treatment: A Retrospective, Multi-Center Study. Front. Pharmacol. 2016, 7, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez-Bonnet, A.; Willis, C.; Pittaway, R.; Smith, K.; Mair, T.; Priestnall, S.L. Molecular carcinogenesis in equine penile cancer: A potential animal model for human penile cancer. Urol. Oncol. 2018, 36, 532.e9–532.e18. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Cheng, X.; Chahoud, J.; Sarhan, A.; Tamboli, P.; Rao, P.; Guo, M.; Manyam, G.; Zhang, L.; Xiang, Y.; et al. Effective combinatorial immunotherapy for penile squamous cell carcinoma. Nat. Commun. 2020, 11, 2124. [Google Scholar] [CrossRef]
- Medeiros-Fonseca, B.; Mestre, V.F.; Estevao, D.; Sanchez, D.F.; Canete-Portillo, S.; Fernandez-Nestosa, M.J.; Casaca, F.; Silva, S.; Brito, H.; Felix, A.; et al. HPV16 induces penile intraepithelial neoplasia and squamous cell carcinoma in transgenic mice: First mouse model for HPV-related penile cancer. J. Pathol. 2020, 251, 411–419. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Deng, C.Z.; Li, Z.S.; Chen, J.P.; Yao, K.; Huang, K.B.; Liu, T.Y.; Liu, Z.W.; Qin, Z.K.; Zhou, F.J.; et al. Molecular characterization and integrative genomic analysis of a panel of newly established penile cancer cell lines. Cell Death Dis. 2018, 9, 684. [Google Scholar] [CrossRef] [Green Version]
- Bleeker, M.C.; Heideman, D.A.; Snijders, P.J.; Horenblas, S.; Dillner, J.; Meijer, C.J. Penile cancer: Epidemiology, pathogenesis and prevention. World J. Urol. 2009, 27, 141–150. [Google Scholar] [CrossRef]
- Cubilla, A.L.; Lloveras, B.; Alejo, M.; Clavero, O.; Chaux, A.; Kasamatsu, E.; Monfulleda, N.; Tous, S.; Alemany, L.; Klaustermeier, J.; et al. Value of p16(INK)(4)(a) in the pathology of invasive penile squamous cell carcinomas: A report of 202 cases. Am. J. Surg. Pathol. 2011, 35, 253–261. [Google Scholar] [CrossRef]
- Bravo, I.G.; Felez-Sanchez, M. Papillomaviruses: Viral evolution, cancer and evolutionary medicine. Evol. Med. Public Health 2015, 2015, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Estevao, D.; Costa, N.R.; Gil da Costa, R.M.; Medeiros, R. Hallmarks of HPV carcinogenesis: The role of E6, E7 and E5 oncoproteins in cellular malignancy. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.O.; Peixoto da Silva, S.; Costa, N.R.; Gil da Costa, R.M.; Medeiros, R. The Role of MicroRNAs in the Metastatic Process of High-Risk HPV-Induced Cancers. Cancers 2018, 10, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbeit, J.M.; Howley, P.M.; Hanahan, D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc. Natl. Acad. Sci. USA 1996, 93, 2930–2935. [Google Scholar] [CrossRef] [Green Version]
- Gil da Costa, R.M.; Neto, T.; Estevao, D.; Moutinho, M.; Felix, A.; Medeiros, R.; Lopes, C.; Bastos, M.; Oliveira, P.A. Ptaquiloside from bracken (Pteridium spp.) promotes oral carcinogenesis initiated by HPV16 in transgenic mice. Food Funct. 2020, 11, 3298–3305. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Soskin, A.; Vieillefond, A.; Carlotti, A.; Plantier, F.; Chaux, A.; Ayala, G.; Velazquez, E.F.; Cubilla, A.L. Warty/basaloid penile intraepithelial neoplasia is more prevalent than differentiated penile intraepithelial neoplasia in nonendemic regions for penile cancer when compared with endemic areas: A comparative study between pathologic series from Paris and Paraguay. Hum. Pathol. 2012, 43, 190–196. [Google Scholar] [CrossRef]
- Widdice, L.E.; Bernstein, D.I.; Franco, E.L.; Ding, L.; Brown, D.R.; Ermel, A.C.; Higgins, L.; Kahn, J.A. Decline in vaccine-type human papillomavirus prevalence in young men from a Midwest metropolitan area of the United States over the six years after vaccine introduction. Vaccine 2019, 37, 6832–6841. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfstrom, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundstrom, K.; Dillner, J.; Sparen, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- da Costa Nunes, J.F.; Pires, S.; Chade, D.C. Human papillomavirus vaccination and prevention of intraepithelial neoplasia and penile cancer: Review article. Curr. Opin. Urol. 2020, 30, 208–212. [Google Scholar] [CrossRef]
- Kuasne, H.; Colus, I.M.; Busso, A.F.; Hernandez-Vargas, H.; Barros-Filho, M.C.; Marchi, F.A.; Scapulatempo-Neto, C.; Faria, E.F.; Lopes, A.; Guimaraes, G.C.; et al. Genome-wide methylation and transcriptome analysis in penile carcinoma: Uncovering new molecular markers. Clin. Epigenetics 2015, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottenhof, S.R.; Djajadiningrat, R.S.; de Jong, J.; Thygesen, H.H.; Horenblas, S.; Jordanova, E.S. Expression of Programmed Death Ligand 1 in Penile Cancer is of Prognostic Value and Associated with HPV Status. J. Urol. 2017, 197, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Ottenhof, S.R.; Djajadiningrat, R.S.; Thygesen, H.H.; Jakobs, P.J.; Jozwiak, K.; Heeren, A.M.; de Jong, J.; Sanders, J.; Horenblas, S.; Jordanova, E.S. The Prognostic Value of Immune Factors in the Tumor Microenvironment of Penile Squamous Cell Carcinoma. Front. Immunol. 2018, 9, 1253. [Google Scholar] [CrossRef] [Green Version]
- Ashley, S.; Shanks, J.H.; Oliveira, P.; Lucky, M.; Parnham, A.; Lau, M.; Sangar, V. Human Papilloma Virus (HPV) status may impact treatment outcomes in patients with pre-cancerous penile lesions (an eUROGEN Study). Int. J. Impot. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Yao, K.; Lu, J.; Zhang, Y.; Chen, K.; Lu, J.; Zhang, C.Z.; Cao, Y. Immunophenotypes Based on the Tumor Immune Microenvironment Allow for Unsupervised Penile Cancer Patient Stratification. Cancers 2020, 12, 1796. [Google Scholar] [CrossRef] [PubMed]
- Yamane, I.; Tsuda, T. Establishment of a cell line in vitro from the lesion of a clinical case of penis cancroid. Tohoku J. Exp. Med. 1966, 88, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingle, A.; Ghim, S.; Joh, J.; Chepkoech, I.; Bennett Jenson, A.; Sundberg, J.P. Novel laboratory mouse papillomavirus (MusPV) infection. Vet. Pathol. 2011, 48, 500–505. [Google Scholar] [CrossRef]
- Ishikawa, S.; Kanoh, S.; Nemoto, S. Establishment of a cell line (TSUS-1) derived from a human squamous cell carcinoma of the penis. Hinyokika Kiyo 1983, 29, 373–376. [Google Scholar]
- Gentile, G.; Giraldo, G.; Stabile, M.; Beth-Giraldo, E.; Lonardo, F.; Kyalwazi, S.K.; Perone, L.; Ventruto, V. Cytogenetic study of a cell line of human penile cancer. Ann. Genet. 1987, 30, 164–169. [Google Scholar]
- Tsukamoto, T. Establishment and characterization of a cell line (KU-8) from squamous cell carcinoma of the penis. Keio J. Med. 1989, 38, 277–293. [Google Scholar] [CrossRef]
- Naumann, C.M.; Sperveslage, J.; Hamann, M.F.; Leuschner, I.; Weder, L.; Al-Najar, A.A.; Lemke, J.; Sipos, B.; Junemann, K.P.; Kalthoff, H. Establishment and characterization of primary cell lines of squamous cell carcinoma of the penis and its metastasis. J. Urol. 2012, 187, 2236–2242. [Google Scholar] [CrossRef]
- Munoz, J.J.; Drigo, S.A.; Kuasne, H.; Villacis, R.A.; Marchi, F.A.; Domingues, M.A.; Lopes, A.; Santos, T.G.; Rogatto, S.R. A comprehensive characterization of cell cultures and xenografts derived from a human verrucous penile carcinoma. Tumor Biol. 2016, 37, 11375–11384. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, K.; Li, Z.; Deng, C.; Wang, L.; Yu, X.; Liang, P.; Xie, Q.; Chen, P.; Qin, Z.; et al. Establishment and characterization of a penile cancer cell line, penl1, with a deleterious TP53 mutation as a paradigm of HPV-negative penile carcinogenesis. Oncotarget 2016, 7, 51687–51698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarhan, A.; Shang, X.; Tamboli, P.; Rao, P.; Pettaway, C.; Wang, A.; DePinho, R.; Lu, X. MP81-07 penile carcinoma: Genetically engineered models for novel therapeutics identification. J. Urol. 2017, 197, e1091. [Google Scholar] [CrossRef]
- van den Top, J.G.; Harkema, L.; Lange, C.; Ensink, J.M.; van de Lest, C.H.; Barneveld, A.; van Weeren, P.R.; Grone, A.; Martens, A. Expression of p53, Ki67, EcPV2- and EcPV3 DNA, and viral genes in relation to metastasis and outcome in equine penile and preputial squamous cell carcinoma. Equine Vet. J. 2015, 47, 188–195. [Google Scholar] [CrossRef]
- Rodriguez, E., Jr.; Weiss, D.A.; Yang, J.H.; Menshenina, J.; Ferretti, M.; Cunha, T.J.; Barcellos, D.; Chan, L.Y.; Risbridger, G.; Cunha, G.R.; et al. New insights on the morphology of adult mouse penis. Biol. Reprod. 2011, 85, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.R.; Wright, D.K.; Gradie, P.E.; Johnston, L.A.; Pask, A.J. A Comprehensive Atlas of the Adult Mouse Penis. Sex. Dev. 2015, 9, 162–172. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, M.M.; DeGraff, D.J.; Yu, X.; Jin, R.J.; Chen, Z.; Borowsky, A.D.; Matusik, R.J. Mouse models of prostate cancer: Picking the best model for the question. Cancer Metastasis Rev. 2014, 33, 377–397. [Google Scholar] [CrossRef]
- Santos, C.; Neto, T.; Ferreirinha, P.; Sousa, H.; Ribeiro, J.; Bastos, M.; Faustino-Rocha, A.I.; Oliveira, P.A.; Medeiros, R.; Vilanova, M.; et al. Celecoxib promotes degranulation of CD8(+) T cells in HPV-induced lesions of K14-HPV16 transgenic mice. Life Sci. 2016, 157, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Polakis, P. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 2007, 17, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Arya, M.; Thrasivoulou, C.; Henrique, R.; Millar, M.; Hamblin, R.; Davda, R.; Aare, K.; Masters, J.R.; Thomson, C.; Muneer, A.; et al. Targets of Wnt/ss-catenin transcription in penile carcinoma. PLoS ONE 2015, 10, e0124395. [Google Scholar] [CrossRef]
- Arbeit, J.M.; Munger, K.; Howley, P.M.; Hanahan, D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J. Virol. 1994, 68, 4358–4368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, L.M.; Hanahan, D.; Arbeit, J.M. Genetic predisposition and parameters of malignant progression in K14-HPV16 transgenic mice. Am. J. Pathol. 1996, 149, 1899–1917. [Google Scholar] [PubMed]
- Stelzer, M.K.; Pitot, H.C.; Liem, A.; Schweizer, J.; Mahoney, C.; Lambert, P.F. A mouse model for human anal cancer. Cancer Prev. Res. (Phila.) 2010, 3, 1534–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strati, K.; Pitot, H.C.; Lambert, P.F. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proc. Natl. Acad. Sci. USA 2006, 103, 14152–14157. [Google Scholar] [CrossRef] [Green Version]
- Mestre, V.F.; Medeiros-Fonseca, B.; Estevao, D.; Casaca, F.; Silva, S.; Felix, A.; Silva, F.; Colaco, B.; Seixas, F.; Bastos, M.M.; et al. HPV16 is sufficient to induce squamous cell carcinoma specifically in the tongue base in transgenic mice. J. Pathol. 2020, 251, 4–11. [Google Scholar] [CrossRef]
- Paiva, I.; Gil da Costa, R.M.; Ribeiro, J.; Sousa, H.; Bastos, M.M.; Faustino-Rocha, A.; Lopes, C.; Oliveira, P.A.; Medeiros, R. MicroRNA-21 expression and susceptibility to HPV-induced carcinogenesis—role of microenvironment in K14-HPV16 mice model. Life Sci. 2015, 128, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.; Ferreirinha, P.; Sousa, H.; Ribeiro, J.; Bastos, M.M.; Neto, T.; Oliveira, P.A.; Medeiros, R.; Vilanova, M.; Gil da Costa, R.M. Ptaquiloside from bracken (Pteridium spp.) inhibits tumour-infiltrating CD8(+) T cells in HPV-16 transgenic mice. Food Chem. Toxicol. 2016, 97, 277–285. [Google Scholar] [CrossRef]
- Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Cooper, T.K.; Hu, J.; Christensen, N.D. Mouse papillomavirus MmuPV1 infects oral mucosa and preferentially targets the base of the tongue. Virology 2016, 488, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Diorio, G.J.; Leone, A.R.; Spiess, P.E. Management of Penile Cancer. Urology 2016, 96, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Berdjis, N.; Meye, A.; Nippgen, J.; Dittert, D.; Hakenberg, O.; Baretton, G.B.; Wirth, M.P. Expression of Ki-67 in squamous cell carcinoma of the penis. BJU Int. 2005, 96, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.G.; Munday, J.S.; Peters, J.; Dunowska, M. Equine penile squamous cell carcinomas are associated with the presence of equine papillomavirus type 2 DNA sequences. Vet. Pathol. 2011, 48, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Arthurs, C.; Suarez-Bonnet, A.; Willis, C.; Xie, B.; Machulla, N.; Mair, T.S.; Cao, K.; Millar, M.; Thrasivoulou, C.; Priestnall, S.L.; et al. Equine penile squamous cell carcinoma: Expression of biomarker proteins and EcPV2. Sci. Rep. 2020, 10, 7863. [Google Scholar] [CrossRef] [PubMed]
- Sykora, S.; Jindra, C.; Hofer, M.; Steinborn, R.; Brandt, S. Equine papillomavirus type 2: An equine equivalent to human papillomavirus 16? Vet. J. 2017, 225, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ramsauer, A.S.; Kubacki, J.; Favrot, C.; Ackermann, M.; Fraefel, C.; Tobler, K. RNA-seq analysis in equine papillomavirus type 2-positive carcinomas identifies affected pathways and potential cancer markers as well as viral gene expression and splicing events. J. Gen. Virol. 2019, 100, 985–998. [Google Scholar] [CrossRef]
- Aydin, A.M.; Chahoud, J.; Adashek, J.J.; Azizi, M.; Magliocco, A.; Ross, J.S.; Necchi, A.; Spiess, P.E. Understanding genomics and the immune environment of penile cancer to improve therapy. Nat. Rev. Urol. 2020, 17, 555–570. [Google Scholar] [CrossRef] [PubMed]

| Cell Lines | Tissue of Origin | HPV Status | Morphology | Other Characteristics | References Publication Year |
|---|---|---|---|---|---|
| First reported penile cancer cell line | Primary tumor | Not reported | Epithelial | Cytogenetic characterization reported | [70] 1966 |
| TSUS-1 | Negative | Not reported | Epithelial | Epithelial morphology, cytogenetic characterization reported, mean doubling time 38 hours | [72] 1983 |
| PCA-5 | Negative | Not reported, human herpesvirus detected | Epithelial | Epithelial morphology, cytogenetic characterization | [73] 1987 |
| KU-8 | Lymph node metastasis | Not reported | Epithelial | Epithelial morphology, cytogenetic characterization reported, mean doubling time 20 hours, EGFR-positive | [74] 1989 |
| Ki-PeCa-L1, Ki-PeCa-P1 | Primary tumor (Ki-PeCa-P1), lymph node metastasis (Ki-PeCa-L1) | Not reported, positive for p16INK4A | Epithelial | Chemokine profiles available | [75] 2012 |
| P5 | Negative | Negative | Epithelial morphology but sarcomatoid when cultured in vivo | Genomic and transcriptomic characterization | [76] 2016 |
| Penl1, Penl2, 149RM, 149RCa, LM156 | lymph node metastases (Penl1, Penl2, LM156), locally recurrent lesion (149RM) scrotal invasion lesion (149RCa) | Negative | Epithelial | penl2 doubling time: 28 hours, 149RM 26 hours, 149RM and 149RCa 26 hours, LM156 34 hours. All cell lines: genomic characterization available, sensitive to cisplatin, resistant to anti-EGFR therapy | [52,77] 2016, 2018 |
| SA1 | C57Bl/6 mouse primary tumor | Negative | Epithelial | Smad4 and Apc null, cisplatin-sensitive. Genomic, methylation and transcriptomic characterization | [50] 2020 |
| SAP1 | C57Bl/6 mouse primary tumor | Negative | Epithelial | Smad4, Apc and Pten null, cisplatin-resistant. Genomic, methylation and transcriptomic characterization | [50] 2020 |
| Species/Strain | HPV Status | Genetic Modifications | Other Characteristics | Reference Publication Year |
|---|---|---|---|---|
| Horse | HPV status: negative but most are EcPV2-positive. | None. | Spontaneous model. Occurs infrequently in horses. Intraepithelial and pre-malignant lesions: papillomatous lesions. Metastasis: yes, to lymph nodes. | [79] 2014 |
| C57Bl/6 mouse | HPV status: negative. | Based on targeted deletion of Apc/Smad4 with or without Pten deletion. | 100% SCC incidence. Pten deletion confers cisplatin resistance. Intraepithelial and pre-malignant lesions: not described. Metastasis: no. | [50] 2020 |
| FVB/N mouse | HPV status: Positive for HPV16. | Based on targeted expression of the entire HPV16 early region. | Requires exposure to DMBA.29.6% SCC incidence. Intraepithelial and pre-malignant lesions: yes, condylomas and penile intraepithelial neoplasia. Metastasis: no. | [51] 2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros-Fonseca, B.; Cubilla, A.; Brito, H.; Martins, T.; Medeiros, R.; Oliveira, P.; Gil da Costa, R.M. Experimental Models for Studying HPV-Positive and HPV-Negative Penile Cancer: New Tools for An Old Disease. Cancers 2021, 13, 460. https://doi.org/10.3390/cancers13030460
Medeiros-Fonseca B, Cubilla A, Brito H, Martins T, Medeiros R, Oliveira P, Gil da Costa RM. Experimental Models for Studying HPV-Positive and HPV-Negative Penile Cancer: New Tools for An Old Disease. Cancers. 2021; 13(3):460. https://doi.org/10.3390/cancers13030460
Chicago/Turabian StyleMedeiros-Fonseca, Beatriz, Antonio Cubilla, Haissa Brito, Tânia Martins, Rui Medeiros, Paula Oliveira, and Rui M. Gil da Costa. 2021. "Experimental Models for Studying HPV-Positive and HPV-Negative Penile Cancer: New Tools for An Old Disease" Cancers 13, no. 3: 460. https://doi.org/10.3390/cancers13030460
APA StyleMedeiros-Fonseca, B., Cubilla, A., Brito, H., Martins, T., Medeiros, R., Oliveira, P., & Gil da Costa, R. M. (2021). Experimental Models for Studying HPV-Positive and HPV-Negative Penile Cancer: New Tools for An Old Disease. Cancers, 13(3), 460. https://doi.org/10.3390/cancers13030460