Hepatic and Extrahepatic Colorectal Metastases Have Discordant Responses to Systemic Therapy. Pathology Data from Patients Undergoing Simultaneous Resection of Multiple Tumor Sites
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Radiological Response
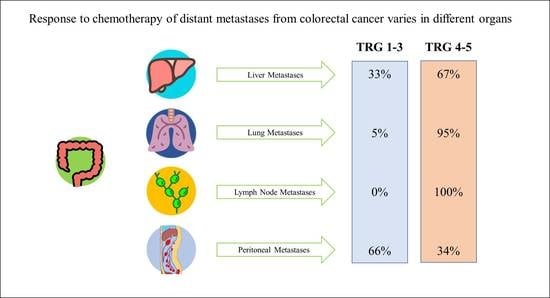
2.2. Pathological Response
2.2.1. Per-Patient Analysis
2.2.2. Per-Lesion Analysis
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; De Gramont, A.; Figueras, J.; Guthrie, A.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; et al. The oncosurgery approach to managing liver metastases from colorectal cancer: A multidisciplinary international consensus. Oncologist 2012, 17, 1225–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 2014, 371, 1609–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasello, G.; Petrelli, F.; Ghidini, M.; Russo, A.; Passalacqua, R.; Barni, S. FOLFOXIRI Plus Bevacizumab as Conversion Therapy for Patients with Initially Unresectable Metastatic Colorectal Cancer: A Systematic Review and Pooled Analysis. JAMA Oncol. 2017, 3, e170278. [Google Scholar] [CrossRef] [PubMed]
- Gruenberger, T.; Bridgewater, J.; Chau, I.; Alfonso, P.G.; Rivoire, M.; Mudan, S.; Lasserre, S.; Hermann, F.; Waterkamp, D.; Adam, R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: The OLIVIA multinational randomised phase II trial. Ann. Oncol. 2015, 26, 702–708. [Google Scholar] [CrossRef]
- Adam, R.; Wicherts, D.A.; de Haas, R.J.; Ciacio, O.; Lévi, F.; Paule, B.; Ducreux, M.; Azoulay, D.; Bismuth, H.; Castaing, D. Patients with initially unresectable colorectal liver metastases: Is there a possibility of cure? J. Clin. Oncol. 2009, 27, 1829–1835. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 2008, 371, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- Viganò, L.; Capussotti, L.; Lapointe, R.; Barroso, E.; Hubert, C.; Giuliante, F.; Ijzermans, J.N.; Mirza, D.F.; Elias, D.; Adam, R. Early recurrence after liver resection for colorectal metastases: Risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann. Surg. Oncol. 2014, 21, 1276–1286. [Google Scholar] [CrossRef]
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Balachandran, V.P.; Kingham, T.P.; De Matteo, R.P.; Allen, P.J.; et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: What factors preclude cure? Surgery 2018, 163, 1238–1244. [Google Scholar] [CrossRef]
- Viganò, L.; Russolillo, N.; Ferrero, A.; Langella, S.; Sperti, E.; Capussotti, L. Evolution of long-term outcome of liver resection for colorectal metastases: Analysis of actual 5-year survival rates over two decades. Ann. Surg. Oncol. 2012, 19, 2035–2044. [Google Scholar] [CrossRef]
- Leung, U.; Gönen, M.; Allen, P.J.; Kingham, T.P.; DeMatteo, R.P.; Jarnagin, W.R.; D’Angelica, M.I. Colorectal Cancer Liver Metastases and Concurrent Extrahepatic Disease Treated with Resection. Ann. Surg. 2017, 265, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; de Haas, R.J.; Wicherts, D.A.; Vibert, E.; Salloum, C.; Azoulay, D.; Castaing, D. Concomitant extrahepatic disease in patients with colorectal liver metastases: When is there a place for surgery? Ann. Surg. 2011, 253, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.; Mentha, G.; Adam, R.; Gerstel, E.; Skipenko, O.G.; Barroso, E.; Lopez-Ben, S.; Hubert, C.; Majno, P.E.; Toso, C. Surgical management of patients with colorectal cancer and simultaneous liver and lung metastases. Br. J. Surg. 2015, 102, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Pedicini, V.; Comito, T.; Carnaghi, C.; Costa, G.; Poretti, D.; Franzese, C.; Personeni, N.; Del Fabbro, D.; Rimassa, L.; et al. Aggressive and Multidisciplinary Local Approach to Iterative Recurrences of Colorectal Liver Metastases. World J. Surg. 2018, 42, 2651–2659. [Google Scholar] [CrossRef]
- Rubbia-Brandt, L.; Giostra, E.; Brezault, C.; Roth, A.D.; Andres, A.; Audard, V.; Sartoretti, P.; Dousset, B.; Majno, P.E.; Soubrane, O.; et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann. Oncol. 2007, 18, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Capussotti, L.; De Rosa, G.; De Saussure, W.O.; Mentha, G.; Rubbia-Brandt, L. Liver resection for colorectal metastases after chemotherapy: Impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann. Surg. 2013, 258, 731–740; discussion 741–742. [Google Scholar] [CrossRef] [PubMed]
- Brouquet, A.; Blot, C.; Allard, M.A.; Lazure, T.; Sebbagh, M.; Gayet, M.; Lewin, M.; Adam, R.; Penna, C.; Sa Cunha, A.; et al. What is the Prognostic Value of a Discordant Radiologic and Pathologic Response in Patients Undergoing Resection of Colorectal Liver Metastases After Preoperative Chemotherapy? Ann. Surg. Oncol. 2020, 27, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Pascal, G.; Castaing, D.; Azoulay, D.; Delvart, V.; Paule, B.; Levi, F.; Bismuth, H. Tumor progression while on chemotherapy: A contraindication to liver resection for multiple colorectal metastases? Ann. Surg. 2004, 240, 1052–1061; discussion 1061–1064. [Google Scholar] [CrossRef]
- Viganò, L.; Capussotti, L.; Barroso, E.; Nuzzo, G.; Laurent, C.; Ijzermans, J.N.; Gigot, J.F.; Figueras, J.; Gruenberger, T.; Mirza, D.F.; et al. Progression while receiving preoperative chemotherapy should not be an absolute contraindication to liver resection for colorectal metastases. Ann. Surg. Oncol. 2012, 19, 2786–2796. [Google Scholar] [CrossRef]
- Vigano, L.; Darwish, S.S.; Rimassa, L.; Cimino, M.; Carnaghi, C.; Donadon, M.; Procopio, F.; Personeni, N.; Del Fabbro, D.; Santoro, A.; et al. Progression of colorectal liver metastases from the end of chemotherapy to resection: A new contraindication to surgery? Ann. Surg. Oncol. 2018, 25, 1676–1685. [Google Scholar] [CrossRef]
- Vigano, L.; Di Tommaso, L.; Mimmo, A.; Sollai, M.; Cimino, M.; Donadon, M.; Roncalli, M.; Torzilli, G. Prospective Evaluation of Intrahepatic Microscopic Occult Tumor Foci in Patients with Numerous Colorectal Liver Metastases. Dig. Surg. 2019, 36, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Baretti, M.; Personeni, N.; Destro, A.; Santoro, A.; Rimassa, L. Emergence of KRAS-mutation in liver metastases after an anti-EGFR treatment in patient with colorectal cancer: Are we aware of the therapeutic impact of intratumor heterogeneity? Cancer Biol. Ther. 2018, 19, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Sebagh, M.; Allard, M.A.; Bosselut, N.; Dao, M.; Vibert, E.; Lewin, M.; Lemoine, A.; Cherqui, D.; Adam, R.; Sa Cunha, A. Evidence of intermetastatic heterogeneity for pathological response and genetic mutations within colorectal liver metastases following preoperative chemotherapy. Oncotarget 2016, 7, 21591–21600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gervaz, P.; Rubbia-Brandt, L.; Andres, A.; Majno, P.; Roth, A.; Morel, P.; Mentha, G. Neoadjuvant chemotherapy in patients with stage IV colorectal cancer: A comparison of histological response in liver metastases, primary tumors, and regional lymph nodes. Ann. Surg. Oncol. 2010, 17, 2714–2719. [Google Scholar] [CrossRef] [PubMed]
- Nanji, S.; Karim, S.; Tang, E.; Brennan, K.; McGuire, A.; Pramesh, C.S.; Booth, C.M. Pulmonary Metastasectomy for Colorectal Cancer: Predictors of Survival in Routine Surgical Practice. Ann. Thorac. Surg. 2018, 105, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampino, M.G.; Maisonneuve, P.; Ravenda, P.S.; Magni, E.; Casiraghi, M.; Solli, P.; Petrella, F.; Gasparri, R.; Galetta, D.; Borri, A.; et al. Lung metastases from colorectal cancer: Analysis of prognostic factors in a single institution study. Ann. Thorac. Surg. 2014, 98, 1238–1245. [Google Scholar] [CrossRef]
- Del Fabbro, D.; Alloisio, M.; Procopio, F.; Cimino, M.; Donadon, M.; Palmisano, A.; Vigano, L.; Torzilli, G. Surgical treatment of synchronous colorectal liver and lung metastases: The usefulness of thoracophrenolaparotomy for single stage resection. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 216–219. [Google Scholar] [CrossRef]
- Brouquet, A.; Vauthey, J.N.; Contreras, C.M.; Walsh, G.L.; Vaporciyan, A.A.; Swisher, S.G.; Curley, S.A.; Mehran, R.J.; Abdalla, E.K. Improved survival after resection of liver and lung colorectal metastases compared with liver-only metastases: A study of 112 patients with limited lung metastatic disease. J. Am. Coll. Surg. 2011, 213, 62–69; discussion 69–71. [Google Scholar] [CrossRef]
- Mise, Y.; Kopetz, S.; Mehran, R.J.; Aloia, T.A.; Conrad, C.; Brudvik, K.W.; Taggart, M.W.; Vauthey, J.N. Is Complete Liver Resection Without Resection of Synchronous Lung Metastases Justified? Ann. Surg. Oncol. 2015, 22, 1585–1592. [Google Scholar] [CrossRef]
- Hagness, M.; Foss, A.; Line, P.D.; Scholz, T.; Jørgensen, P.F.; Fosby, B.; Boberg, K.M.; Mathisen, O.; Gladhaug, I.P.; Egge, T.S.; et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann. Surg. 2013, 257, 800–806. [Google Scholar] [CrossRef]
- Dueland, S.; Guren, T.K.; Hagness, M.; Glimelius, B.; Line, P.D.; Pfeiffer, P.; Foss, A.; Tveit, K.M. Chemotherapy or liver transplantation for nonresectable liver metastases from colorectal cancer? Ann. Surg. 2015, 261, 956–960. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Qin, Y. Peri-operative chemotherapy for resectable colorectal lung metastasis: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2020, 146, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghidini, M.; Personeni, N.; Bozzarelli, S.; Baretti, M.; Basso, G.; Bianchi, P.; Tronconi, M.C.; Pressiani, T.; Grizzi, F.; Giordano, L.; et al. KRAS mutation in lung metastases from colorectal cancer: Prognostic implications. Cancer Med. 2016, 5, 256–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, R.; de Haas, R.J.; Wicherts, D.A.; Aloia, T.A.; Delvart, V.; Azoulay, D.; Bismuth, H.; Castaing, D. Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J. Clin. Oncol. 2008, 26, 3672–3680. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Waite, K.; Youssef, H. The Role of Neoadjuvant and Adjuvant Systemic Chemotherapy with Cytoreductive Surgery and Heated Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Systematic Review. Ann. Surg. Oncol. 2017, 24, 705–720. [Google Scholar] [CrossRef]
- Cashin, P.H.; Mahteme, H.; Spång, N.; Syk, I.; Frödin, J.E.; Torkzad, M.; Glimelius, B.; Graf, W. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: A randomised trial. Eur. J. Cancer 2016, 53, 155–162. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, in press. [Google Scholar] [CrossRef]
- Klement, R.J.; Abbasi-Senger, N.; Adebahr, S.; Alheid, H.; Allgaeuer, M.; Becker, G.; Blanck, O.; Boda-Heggemann, J.; Brunner, T.; Duma, M.; et al. The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: A combined analysis of 388 patients with 500 metastases. BMC Cancer 2019, 19, 173. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Torzilli, G.; Viganò, L.; Gatti, A.; Costa, G.; Cimino, M.; Procopio, F.; Donadon, M.; Del Fabbro, D. Twelve-year experience of “radical but conservative” liver surgery for colorectal metastases: Impact on surgical practice and oncologic efficacy. HPB (Oxford) 2017, 19, 775–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viganò, L.; Procopio, F.; Cimino, M.M.; Donadon, M.; Gatti, A.; Costa, G.; Del Fabbro, D.; Torzilli, G. Is Tumor Detachment from Vascular Structures Equivalent to R0 Resection in Surgery for Colorectal Liver Metastases? An Observational Cohort. Ann. Surg. Oncol. 2016, 23, 1352–1360. [Google Scholar] [CrossRef] [PubMed]

| Demographical Characteristics | n = 45 | |
| Age, years, median (range) | 59 (34–76) | |
| Sex (M:F) | 27 (60%):18 (40%) | |
| Primary tumor site (right colon: left colon: rectum) | 15 (33%):17 (38%):13 (29%) | |
| Microsatellite instability | 0 | |
| Synchronous liver metastases | 31 (69%) | |
| Number of liver metastases, median (range) | 2 (1–10) | |
| Size of liver metastases, mm, median (range) | 14 (1–180) | |
| KRAS mutated status (status available in 36 patients) | 20/36 (56%) | |
| NRAS mutated status (status available in 27 patients) | 0 | |
| BRAF mutated status (status available in 26 patients) | 0 | |
| Extrahepatic disease | ||
| Lung | 15 | |
| Number of nodules, median (range) | 1 (1–3) | |
| Size, mm, median (range) | 7 (3–37) | |
| Lymph nodes | 14 | |
| Number of nodules, median (range) | 1 (1–2) | |
| Size, mm, median (range) | 15 (10–40) | |
| Peritoneum | 21 | |
| Number of nodules, median (range) | 1 (1–5) | |
| Size, mm, median (range) | 9 (2–40) | |
| Adrenal gland | 1 | |
| Chemotherapy details | ||
| Regimen | ||
| Oxaliplatin | 22 (49%) | |
| Irinotecan | 21 (47%) | |
| Oxaliplatin + Irinotecan | 2 (4%) | |
| Targeted therapies | 27 (60%) | |
| Anti-VEGF | 19 (42%) | |
| Anti-EGFR | 8 (18%) | |
| Number of cycles, median (range) | 7 (4–24) | |
| Number of lines, median (range) | 1 (1–2) | |
| Per-Patient Analysis (n = 25) | |||||
|---|---|---|---|---|---|
| Liver n = 25 | Extrahepatic n = 25 | Lung n = 13 | Lymph Node n = 9 | Peritoneum n = 7 | |
| Complete response | - | 1 (4%) | - | - | 1 (14%) |
| Partial response | 19 (76%) | 10 (40%) | 3 (23%) | 5 (56%) | 2 (29%) |
| Stable disease | 6 (24%) | 13 (52%) | 9 (69%) | 4 (44%) | 4 (57%) |
| Disease progression | - | 1 (4%) | 1 (8%) | - | - |
| Per-patient Analysis (n = 45) | |||||
|---|---|---|---|---|---|
| TRG | Liver n = 45 | Extrahepatic n = 45 | Lung n = 15 | Lymph Node n = 14 | Peritoneum n = 21 |
| TRG 1–2 | 2 (5%) | 5 (11%) | - | - | 4 (19%) |
| TRG 3 | 6 (13%) | 5 (11%) | - | - | 9 (43%) |
| TRG 4–5 | 37 (82%) | 35 (78%) | 15 (100%) | 14 (100%) | 8 (38%) |
| Per-Lesion Analysis (n = 134 Liver Metastases and n = 72 Extrahepatic Metastases) | |||||
| TRG | Liver n = 134 | Extrahepatic n = 72 | Lung n = 21 | Lymph node n = 15 | Peritoneum n = 35 |
| TRG 1–2 | 14 (11%) | 7 (10%) | - | - | 7 (20%) |
| TRG 3 | 30 (22%) | 17 (24%) | 1 (5%) | - | 16 (46%) |
| TRG 4–5 | 90 (67%) | 48 (67%) | 20 (95%) | 15 (100%) | 12 (34%) |
| Parameter | TRG 1–3 | Univariate | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| p | OR | 95% CI | ||||
| Metastasis site | Liver | 44 (32.8%) | <0.001 | 1 | ||
| Lung | 1 (4.8%) | 0.014 | 0.057 | 0.006–0.566 | ||
| Lymph node | - (0%) | Omitted (perfect prediction of failure) | ||||
| Peritoneum | 23 (65.7%) | <0.001 | 12.709 | 3.102–52.063 | ||
| Adrenal | - (0%) | Omitted (perfect prediction of failure) | ||||
| Metastasis size, mm | 13.9 ± 12.9 (vs. 20.4 ± 26.3) | 0.023 | 0.049 | 0.961 | 0.923–0.999 | |
| Primary tumor site | Right colon | 28 (43.1%) | 0.018 | 1 | ||
| Left colon | 32 (33.7%) | 0.635 | 0.774 | 0.270–2.223 | ||
| Rectum | 8 (17.4%) | 0.196 | 0.364 | 0.078–1.687 | ||
| KRAS status * | Wild type | 19 (29.7%) | 0.101 | 0.218 | 0.385 | 0.084–1.757 |
| Mutated | 40 (42.6%) | |||||
| Oxaliplatin | Y | 25 (28.7%) | 0.265 | 0.178 | 8.533 | 0.376–193.728 |
| n | 43 (36.1%) | |||||
| Irinotecan | Y | 44 (36.4%) | 0.222 | 0.151 | 9.905 | 0.432–226.851 |
| N | 24 (28.2%) | |||||
| Anti-VEGF | Y | 33 (39.3%) | 0.112 | 0.001 | 9.748 | 2.498–38.041 |
| N | 35 (28.7%) | |||||
| Anti-EGFR | Y | 23 (56.1%) | <0.001 | <0.001 | 69.830 | 8.977–543.186 |
| N | 45 (27.3%) | |||||
| Number of lines | 1 | 61 (33.0%) | 0.973 | 0.402 | 1.927 | 0.416–8.922 |
| >1 | 7 (33.3%) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigano, L.; Corleone, P.; Darwish, S.S.; Turri, N.; Famularo, S.; Viggiani, L.; Rimassa, L.; Del Fabbro, D.; Di Tommaso, L.; Torzilli, G. Hepatic and Extrahepatic Colorectal Metastases Have Discordant Responses to Systemic Therapy. Pathology Data from Patients Undergoing Simultaneous Resection of Multiple Tumor Sites. Cancers 2021, 13, 464. https://doi.org/10.3390/cancers13030464
Vigano L, Corleone P, Darwish SS, Turri N, Famularo S, Viggiani L, Rimassa L, Del Fabbro D, Di Tommaso L, Torzilli G. Hepatic and Extrahepatic Colorectal Metastases Have Discordant Responses to Systemic Therapy. Pathology Data from Patients Undergoing Simultaneous Resection of Multiple Tumor Sites. Cancers. 2021; 13(3):464. https://doi.org/10.3390/cancers13030464
Chicago/Turabian StyleVigano, Luca, Pio Corleone, Shadya Sara Darwish, Nicolò Turri, Simone Famularo, Lorenzo Viggiani, Lorenza Rimassa, Daniele Del Fabbro, Luca Di Tommaso, and Guido Torzilli. 2021. "Hepatic and Extrahepatic Colorectal Metastases Have Discordant Responses to Systemic Therapy. Pathology Data from Patients Undergoing Simultaneous Resection of Multiple Tumor Sites" Cancers 13, no. 3: 464. https://doi.org/10.3390/cancers13030464
APA StyleVigano, L., Corleone, P., Darwish, S. S., Turri, N., Famularo, S., Viggiani, L., Rimassa, L., Del Fabbro, D., Di Tommaso, L., & Torzilli, G. (2021). Hepatic and Extrahepatic Colorectal Metastases Have Discordant Responses to Systemic Therapy. Pathology Data from Patients Undergoing Simultaneous Resection of Multiple Tumor Sites. Cancers, 13(3), 464. https://doi.org/10.3390/cancers13030464