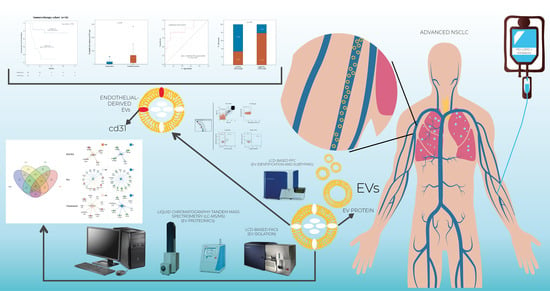
Phenotypic and Proteomic Analysis Identifies Hallmarks of Blood Circulating Extracellular Vesicles in NSCLC Responders to Immune Checkpoint Inhibitors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Collection
2.3. Flow Cytometry Detection of Extracellular Vesicles
2.4. Extracellular Vesicle Identification and Subtyping
2.5. Extracellular Vesicle Isolation by Fluorescence-Activated Cell Sorting
2.6. Label-Free Proteomics of Circulating EVs
2.7. Proteomics Data Processing
2.8. Bioinformatics Analysis
2.9. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. EVs Frequencies
3.3. Circulating Endothelial-EV Concentration Is Associated with Overall Survival
3.4. Circulating Endothelial-EV Concentration Is Associated with Disease Control Rate
3.5. Proteomic Analysis Reveals Specific Protein Cargo in Responders vs. Non Responders
3.6. Anti-PD1 Treatment Modulates a Subset of EV Proteins Involved in Immune Function
3.7. Anti-PD-1 Treatment Modulates Pathways Involved in Immune Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Allophycocyanin |
| AUC | Curve with corresponding area under the curve |
| CR | Complete response |
| DCR | Disease control rate |
| EV | Extracellular vesicle |
| FASP | Filter-aided sample preparation |
| FMO | Fluorescence minus one |
| iBAQ | Intensity-based absolute quantification |
| ICI | Immune checkpoint inhibitor |
| IDO | Indoleamine 2,3-dioxygenase |
| IPA | Ingenuity Pathway Analysis |
| LC-MS/MS | Liquid chromatography tandem mass spectrometry |
| LCD | Lipophilic cationic dye |
| MBR | Match-between-runs |
| mOS | Median overall survival |
| NRB | Non-Responders at baseline |
| NRP | Non-responders post-treatment |
| NSCLC | Non-small cell lung cancer |
| PD-1 | Programmed cell death 1 |
| PD-L1 | Programmed cell death-ligand 1 |
| PD | Progressive disease |
| PECAM-1 | Platelet endothelial adhesion molecule-1 |
| PFC | Polychromatic flow cytometry |
| PR | Partial response |
| RB | Responders at baseline |
| ROC | Receiving operator characteristic |
| RP | Responders post-treatment; |
| SD | Stable disease; |
| TME | Tumor microenvironment; |
| URA | Upstream Regulator Analysis; |
| VEGF | Vascular endothelial growth factor; |
References
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in Non–Small Cell Lung Cancer: Facts and Hopes. Clin. Cancer Res. 2019, 25, 4592–4602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowicki, T.S.; Hu-Lieskovan, S.; Ribas, A. Mechanisms of Resistance to PD-1 and PD-L1 Blockade. Cancer J. 2018, 24, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Doebele, R.C.; Kerr, K.M. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat. Rev. Clin. Oncol. 2019, 16, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Bassanelli, M.; Sioletic, S.; Martini, M.; Giacinti, S.; Viterbo, A.; Staddon, A.; Liberati, F.; Ceribelli, A. Heterogeneity of PD-L1 Expression and Relationship with Biology of NSCLC. Anticancer Res. 2018, 38, 3789–3796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cufaro, M.C.; Pieragostino, D.; Lanuti, P.; Rossi, C.; Cicalini, I.; Federici, L.; De Laurenzi, V.; Del Boccio, P. Extracellular Vesicles and Their Potential Use in Monitoring Cancer Progression and Therapy: The Contribution of Proteomics. J. Oncol. 2019, 2019, 1639854. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Pasini, L.; Ulivi, P. Extracellular Vesicles in Non-Small-Cell Lung Cancer: Functional Role and Involvement in Resistance to Targeted Treatment and Immunotherapy. Cancers 2019, 12, 40. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Lam, E.W.-F.; Sun, Y. Extracellular vesicles in the tumor microenvironment: Old stories, but new tales. Mol. Cancer 2019, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Poggio, M.; Hu, T.; Pai, C.-C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 2019, 177, 414–427.e13. [Google Scholar] [CrossRef] [Green Version]
- Gärtner, K.; Battke, C.; Dünzkofer, J.; Hüls, C.; von Neubeck, B.; Kellner, M.-K.; Fiestas, E.; Fackler, S.; Lang, S.; Zeidler, R. Tumor-derived extracellular vesicles activate primary monocytes. Cancer Med. 2018, 7, 2013–2020. [Google Scholar] [CrossRef]
- Lindenbergh, M.F.S.; Stoorvogel, W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu. Rev. Immunol. 2018, 36, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Shukuya, T.; Ghai, V.; Amann, J.M.; Okimoto, T.; Shilo, K.; Kim, T.-K.; Wang, K.; Carbone, D.P. Circulating MicroRNAs and Extracellular Vesicle–Containing MicroRNAs as Response Biomarkers of Anti–programmed Cell Death Protein 1 or Programmed Death-Ligand 1 Therapy in NSCLC. J. Thorac. Oncol. 2020, 15, 1773–1781. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Re, M.; Marconcini, R.; Pasquini, G.; Rofi, E.; Vivaldi, C.; Bloise, F.; Restante, G.; Arrigoni, E.; Caparello, C.; Bianco, M.G.; et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br. J. Cancer 2018, 118, 820–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simeone, P.; Celia, C.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Cilurzo, F.; Grande, R.; Diomede, F.; Vespa, S.; Canonico, B.; et al. Diameters and Fluorescence Calibration for Extracellular Vesicle Analyses by Flow Cytometry. Int. J. Mol. Sci. 2020, 21, 7885. [Google Scholar] [CrossRef]
- Marchisio, M.; Simeone, P.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Pieragostino, D.; Ventrella, A.; Antonini, F.; Del Zotto, G.; Vergara, D.; et al. Flow Cytometry Analysis of Circulating Extracellular Vesicle Subtypes from Fresh Peripheral Blood Samples. Int. J. Mol. Sci. 2020, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Brocco, D.; Lanuti, P.; Simeone, P.; Bologna, G.; Pieragostino, D.; Cufaro, M.C.; Graziano, V.; Peri, M.; Di Marino, P.; De Tursi, M.; et al. Circulating Cancer Stem Cell-Derived Extracellular Vesicles as a Novel Biomarker for Clinical Outcome Evaluation. J. Oncol. 2019, 2019, 5879616. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Cicalini, I.; Cufaro, M.C.; Agnifili, L.; Mastropasqua, L.; Lanuti, P.; Marchisio, M.; De Laurenzi, V.; Del Boccio, P.; Pieragostino, D. Multi-Omics Approach for Studying Tears in Treatment-Naïve Glaucoma Patients. Int. J. Mol. Sci. 2019, 20, 4029. [Google Scholar] [CrossRef] [Green Version]
- Pieragostino, D.; Lanuti, P.; Cicalini, I.; Cufaro, M.C.; Ciccocioppo, F.; Ronci, M.; Simeone, P.; Onofrj, M.; van der Pol, E.; Fontana, A.; et al. Proteomics characterization of extracellular vesicles sorted by flow cytometry reveals a disease-specific molecular cross-talk from cerebrospinal fluid and tears in multiple sclerosis. J. Proteom. 2019, 204, 103403. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, J.P.; Jones, J.C. Detection of platelet vesicles by flow cytometry. Platelets 2017, 28, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Falasca, K.; Lanuti, P.; Ucciferri, C.; Pieragostino, D.; Cufaro, M.C.; Bologna, G.; Federici, L.; Miscia, S.; Pontolillo, M.; Auricchio, A.; et al. Circulating extracellular vesicles as new inflammation marker in hiv infection. AIDS 2020. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieragostino, D.; Cicalini, I.; Lanuti, P.; Ercolino, E.; di Ioia, M.; Zucchelli, M.; Zappacosta, R.; Miscia, S.; Marchisio, M.; Sacchetta, P.; et al. Enhanced release of acid sphingomyelinase-enriched exosomes generates a lipidomics signature in CSF of Multiple Sclerosis patients. Sci. Rep. 2018, 8, 3071. [Google Scholar] [CrossRef] [Green Version]
- Tyanova, S.; Cox, J. Perseus: A Bioinformatics Platform for Integrative Analysis of Proteomics Data in Cancer Research. In Cancer Systems Biology; Humana Press: New York, NY, USA, 2018; pp. 133–148. [Google Scholar]
- Berghmans, T.; Durieux, V.; Hendriks, L.E.L.; Dingemans, A.-M. Immunotherapy: From Advanced NSCLC to Early Stages, an Evolving Concept. Front. Med. 2020, 7, 90. [Google Scholar] [CrossRef] [Green Version]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Nixon, A.B.; Schalper, K.A.; Jacobs, I.; Potluri, S.; Wang, I.-M.; Fleener, C. Peripheral immune-based biomarkers in cancer immunotherapy: Can we realize their predictive potential? J. Immunother. Cancer 2019, 7, 325. [Google Scholar] [CrossRef]
- Ma, L.; Mauro, C.; Cornish, G.H.; Chai, J.-G.; Coe, D.; Fu, H.; Patton, D.; Okkenhaug, K.; Franzoso, G.; Dyson, J.; et al. Ig gene-like molecule CD31 plays a nonredundant role in the regulation of T-cell immunity and tolerance. Proc. Natl. Acad. Sci. USA 2010, 107, 19461–19466. [Google Scholar] [CrossRef] [Green Version]
- Clement, M.; Fornasa, G.; Guedj, K.; Ben Mkaddem, S.; Gaston, A.-T.; Khallou-Laschet, J.; Morvan, M.; Nicoletti, A.; Caligiuri, G. CD31 is a key coinhibitory receptor in the development of immunogenic dendritic cells. Proc. Natl. Acad. Sci. USA 2014, 111, E1101–E1110. [Google Scholar] [CrossRef] [Green Version]
- Motz, G.T.; Coukos, G. The parallel lives of angiogenesis and immunosuppression: Cancer and other tales. Nat. Rev. Immunol. 2011, 11, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Tanaka, T.; Akasaki, Y.; Murayama, Y.; Yoshida, K.; Sasaki, H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: Perspectives for therapeutic implications. Med. Oncol. 2019, 37, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffioen, A.W.; Damen, C.A.; Blijham, G.H.; Groenewegen, G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood 1996, 88, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Dirkx, A.E.M.; Oude Egbrink, M.G.A.; Kuijpers, M.J.E.; van der Niet, S.T.; Heijnen, V.V.T.; Bouma-ter Steege, J.C.A.; Wagstaff, J.; Griffioen, A.W. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 2003, 63, 2322–2329. [Google Scholar] [PubMed]
- Zhang, J.; Chen, C.; Hu, B.; Niu, X.; Liu, X.; Zhang, G.; Zhang, C.; Li, Q.; Wang, Y. Exosomes Derived from Human Endothelial Progenitor Cells Accelerate Cutaneous Wound Healing by Promoting Angiogenesis Through Erk1/2 Signaling. Int. J. Biol. Sci. 2016, 12, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.; Dentelli, P.; Togliatto, G.; Rosso, A.; Gili, M.; Gallo, S.; Deregibus, M.C.; Camussi, G.; Brizzi, M.F. Activated Stat5 trafficking Via Endothelial Cell-derived Extracellular Vesicles Controls IL-3 Pro-angiogenic Paracrine Action. Sci. Rep. 2016, 6, 25689. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef]
- Wagner, N.B.; Weide, B.; Gries, M.; Reith, M.; Tarnanidis, K.; Schuermans, V.; Kemper, C.; Kehrel, C.; Funder, A.; Lichtenberger, R.; et al. Tumor microenvironment-derived S100A8/A9 is a novel prognostic biomarker for advanced melanoma patients and during immunotherapy with anti-PD-1 antibodies. J. Immunother. Cancer 2019, 7, 343. [Google Scholar] [CrossRef]
- Ortiz, M.L.; Lu, L.; Ramachandran, I.; Gabrilovich, D.I. Myeloid-Derived Suppressor Cells in the Development of Lung Cancer. Cancer Immunol. Res. 2014, 2, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.-W.; Liu, Y.-F.; Cao, X.-Q.; Wang, Y.; Cui, X.-H.; Ye, X.; Huang, S.-W.; Xie, H.-J.; Zhang, H.-J. Annexin A2 promotion of hepatocellular carcinoma tumorigenesis via the immune microenvironment. World J. Gastroenterol. 2020, 26, 2126–2137. [Google Scholar] [CrossRef]
- Brichory, F.M.; Misek, D.E.; Yim, A.-M.; Krause, M.C.; Giordano, T.J.; Beer, D.G.; Hanash, S.M. An immune response manifested by the common occurrence of annexins I and II autoantibodies and high circulating levels of IL-6 in lung cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 9824–9829. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Univariate | Bootstrap Results (1000 Replicas) | Multivariate 1 | Bootstrap Results (1000 Replicas) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Groups. | HR (95% CI) | p | Bias | SE | 95% CI | p | HR (95% CI) | p | Bias | SE | 95% CI | p |
| Total EVs | ≤14,360 EVs/μL vs. >14,360 EVs/μL2 | 0.45 (0.17–1.14) | 0.09 | −0.003 | 0.43 | −1.70 to 0.01 | 0.04 | ||||||
| Leukocyte-EVs | ≤169 EVs/μL vs.>169 EVs/μL 2 | 1.19 (0.26–2.01) | 0.76 | −0.01 3 | 0.72 3 | −1.18 to 1.37 3 | 0.72 3 | ||||||
| Endothelial-EVs | ≤94 EVs/μL vs. >94 EVs/μL 2 | 0.13 (0.04–0.50) | 0.003 | −0.19 | 0.91 | −4.77 to −0.87 | 0.004 | 0.16 (0.04–0.63) | 0.008 | −0.96 | 3.10 | −13.4 to −0.65 | 0.005 |
| Age | ≥65 vs. <65 | 1.24 (0.49–3.10) | 0.65 | 0.06 | 0.47 | −0.64 to 1.26 | 0.61 | ||||||
| No. metastatic sites | ≥2 vs. <2 | 2.86 (1.02–8.04) | 0.04 | 0.11 | 0.57 | 0.21 to 2.42 | 0.02 | 2.67 (0.73–9.70) | 0.13 | 0.58 | 2.30 | 0.08 to 12.3 | 0.04 |
| ECOG PS | 1–2 vs. 0 | 2.77 (0.90–8.54) | 0.08 | 0.14 | 0.64 | 0.19 to 2.66 | 0.02 | ||||||
| Tissue PD-L1 | ≥1% vs. <1% | 0.77 (0.42–1.45) | 0.43 | −0.13 | 0.48 | −1.67 to 0.30 | 0.45 | ||||||
| Line of therapy | 2nd/3rd line vs. 1st line | 1.18 (0.55–2.56) | 0.66 | −0.007 | 0.40 | −0.70 to 0.97 | 0.40 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brocco, D.; Lanuti, P.; Pieragostino, D.; Cufaro, M.C.; Simeone, P.; Bologna, G.; Di Marino, P.; De Tursi, M.; Grassadonia, A.; Irtelli, L.; et al. Phenotypic and Proteomic Analysis Identifies Hallmarks of Blood Circulating Extracellular Vesicles in NSCLC Responders to Immune Checkpoint Inhibitors. Cancers 2021, 13, 585. https://doi.org/10.3390/cancers13040585
Brocco D, Lanuti P, Pieragostino D, Cufaro MC, Simeone P, Bologna G, Di Marino P, De Tursi M, Grassadonia A, Irtelli L, et al. Phenotypic and Proteomic Analysis Identifies Hallmarks of Blood Circulating Extracellular Vesicles in NSCLC Responders to Immune Checkpoint Inhibitors. Cancers. 2021; 13(4):585. https://doi.org/10.3390/cancers13040585
Chicago/Turabian StyleBrocco, Davide, Paola Lanuti, Damiana Pieragostino, Maria Concetta Cufaro, Pasquale Simeone, Giuseppina Bologna, Pietro Di Marino, Michele De Tursi, Antonino Grassadonia, Luciana Irtelli, and et al. 2021. "Phenotypic and Proteomic Analysis Identifies Hallmarks of Blood Circulating Extracellular Vesicles in NSCLC Responders to Immune Checkpoint Inhibitors" Cancers 13, no. 4: 585. https://doi.org/10.3390/cancers13040585
APA StyleBrocco, D., Lanuti, P., Pieragostino, D., Cufaro, M. C., Simeone, P., Bologna, G., Di Marino, P., De Tursi, M., Grassadonia, A., Irtelli, L., De Lellis, L., Veschi, S., Florio, R., Federici, L., Marchisio, M., Miscia, S., Cama, A., Tinari, N., & Del Boccio, P. (2021). Phenotypic and Proteomic Analysis Identifies Hallmarks of Blood Circulating Extracellular Vesicles in NSCLC Responders to Immune Checkpoint Inhibitors. Cancers, 13(4), 585. https://doi.org/10.3390/cancers13040585