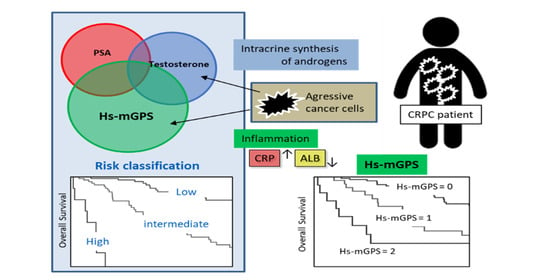
Prognostic Value of High-Sensitivity Modified Glasgow Prognostic Score in Castration-Resistant Prostate Cancer Patients Who Received Docetaxel
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Variables
2.2. High-Sensitivity Modified Glasgow Prognostic Score
2.3. Docetaxel Treatment
2.4. Endpoints and Definition of Progression
2.5. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Survival Analysis Using the Kaplan–Meier Methods in CRPC Patients According to Hs-mGPS
3.3. The Predictive Role of the Hs-mGPS in the Survival Outcome of CRPC Patients
3.4. The Characteristics of the High Hs-mGPS Patients
3.5. Combined Score and Risk Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [Green Version]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef] [Green Version]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foroughi Moghadam, M.J.; Taheri, S.; Peiravian, F. A Systematic Review of Clinical Practice Guidelines for Castration-Resistant Prostate Cancer. Iran. J. Pharm. Res. 2018, 17, 17–37. [Google Scholar] [PubMed]
- Teo, M.Y.; Rathkopf, D.E.; Kantoff, P. Treatment of Advanced Prostate Cancer. Annu. Rev. Med. 2019, 70, 479–499. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Puhr, M. Interleukin-6: A multifunctional targetable cytokine in human prostate cancer. Mol. Cell Endocrinol. 2012, 360, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Lou, W.; Nadiminty, N.; Lin, X.; Gao, A.C. Requirement for NF-(kappa)B in interleukin-4-induced androgen receptor activation in prostate cancer cells. Prostate 2005, 64, 160–167. [Google Scholar] [CrossRef]
- Shen, H.; Schuster, R.; Lu, B.; Waltz, S.E.; Lentsch, A.B. Critical and opposing roles of the chemokine receptors CXCR2 and CXCR3 in prostate tumor growth. Prostate 2006, 66, 1721–1728. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Koch, A.E.; Zhang, J.; Taichman, R.S. CXCR6 induces prostate cancer progression by the AKT/mammalian target of rapamycin signaling pathway. Cancer Res. 2008, 68, 10367–10376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.K.; Lokeshwar, B.L. The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth. Cancer Res. 2011, 71, 3268–3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dunlop, D.J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 2003, 89, 1028–1030. [Google Scholar] [CrossRef] [Green Version]
- McMillan, D.C.; Crozier, J.E.; Canna, K.; Angerson, W.J.; McArdle, C.S. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int. J. Colorectal. Dis. 2007, 22, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.J.; Horgan, P.G.; Talwar, D.; Fletcher, C.D.; Morrison, D.S.; McMillan, D.C. Optimization of the systemic inflammation-based Glasgow prognostic score: A Glasgow Inflammation Outcome Study. Cancer 2013, 119, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Shafique, K.; Proctor, M.J.; McMillan, D.C.; Qureshi, K.; Leung, H.; Morrison, D.S. Systemic inflammation and survival of patients with prostate cancer: Evidence from the Glasgow Inflammation Outcome Study. Prostate Cancer Prostatic Dis. 2012, 15, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Linton, A.; Pond, G.; Clarke, S.; Vardy, J.; Galsky, M.; Sonpavde, G. Glasgow prognostic score as a prognostic factor in metastatic castration-resistant prostate cancer treated with docetaxel-based chemotherapy. Clin. Genitourin. Cancer 2013, 11, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Stangl-Kremser, J.; Mari, A.; Suarez-Ibarrola, R.; D’Andrea, D.; Korn, S.M.; Pones, M.; Kramer, G.; Karakiewicz, P.; Enikeev, D.V.; Glybochko, P.V.; et al. Development of a prognostic model for survival time prediction in castration-resistant prostate cancer patients. Urol. Oncol. 2020, 38, 600.e9–600.e15. [Google Scholar] [CrossRef]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Shafique, K.; Proctor, M.J.; McMillan, D.C.; Leung, H.; Smith, K.; Sloan, B.; Morrison, D.S. The modified Glasgow prognostic score in prostate cancer: Results from a retrospective clinical series of 744 patients. BMC. Cancer 2013, 13, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanai, N.; Sawabe, M.; Kimura, T.; Suzuki, H.; Ozawa, T.; Hirakawa, H.; Fukuda, Y.; Hasegawa, Y. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor for head and neck cancer. Oncotarget 2018, 9, 37008–37016. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Fang, M.; Wan, Q.; Zhang, X.; Song, T.; Wu, S. High-sensitivity modified Glasgow prognostic score (HS-mGPS) Is superior to the mGPS in esophageal cancer patients treated with chemoradiotherapy. Oncotarget 2017, 8, 99861–99870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, T.; Guo, T.; Nie, R.; Hong, D.; Zhou, Z.; Zhang, X.; Liang, Y. The prognostic role of the preoperative systemic immune-inflammation index and high-sensitivity modified Glasgow prognostic score in patients after radical operation for soft tissue sarcoma. Eur. J. Surg. Oncol. 2020, 46, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Lokeshwar, B.L. Depletion of intrinsic expression of Interleukin-8 in prostate cancer cells causes cell cycle arrest, spontaneous apoptosis and increases the efficacy of chemotherapeutic drugs. Mol. Cancer 2009, 8, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingo-Domenech, J.; Oliva, C.; Rovira, A.; Codony-Servat, J.; Bosch, M.; Filella, X.; Montagut, C.; Tapia, M.; Campás, C.; Dang, L.; et al. Interleukin 6, a nuclear factor-kappaB target, predicts resistance to docetaxel in hormone-independent prostate cancer and nuclear factor-kappaB inhibition by PS-1145 enhances docetaxel antitumor activity. Clin. Cancer Res. 2006, 12, 5578–5586. [Google Scholar] [CrossRef] [Green Version]
- Loberg, R.D.; Day, L.L.; Harwood, J.; Ying, C.; St John, L.N.; Giles, R.; Neeley, C.K.; Pienta, K.J. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia 2006, 8, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Qian, D.Z.; Rademacher, B.L.; Pittsenbarger, J.; Huang, C.Y.; Myrthue, A.; Higano, C.S.; Garzotto, M.; Nelson, P.S.; Beer, T.M. CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxel-induced cytotoxicity. Prostate 2010, 70, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roca, H.; Varsos, Z.S.; Pienta, K.J. CCL2 is a negative regulator of AMP-activated protein kinase to sustain mTOR complex-1 activation, survivin expression, and cell survival in human prostate cancer PC3 cells. Neoplasia 2009, 11, 1309–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubik, A.A.; Gunter, J.H.; Hollier, B.G.; Ettinger, S.; Fazli, L.; Stylianou, N.; Hendy, S.C.; Adomat, H.H.; Gleave, M.E.; Pollak, M.; et al. IGF2 increases de novo steroidogenesis in prostate cancer cells. Endocr. Relat. Cancer 2013, 20, 173–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, J.Y.; Nadiminty, N.; Dutt, S.; Lou, W.; Yang, J.C.; Kung, H.J.; Evans, C.P.; Gao, A.C. Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clin. Cancer Res. 2009, 15, 4815–4822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mout, L.; de Wit, R.; Stuurman, D.; Verhoef, E.; Mathijssen, R.; de Ridder, C.; Lolkema, M.; van Weerden, W. Testosterone Diminishes Cabazitaxel Efficacy and Intratumoral Accumulation in a Prostate Cancer Xenograft Model. EBioMedicine 2018, 27, 182–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Sakamoto, S.; Minhui, X.; Tamura, T.; Otsuka, K.; Sato, K.; Maimaiti, M.; Kamada, S.; Takei, A.; Fuse, M.; et al. Testosterone Reduction of ≥ 480 ng/dL Predicts Favorable Prognosis of Japanese Men with Advanced Prostate Cancer Treated With Androgen-Deprivation Therapy. Clin. Genitourin. Cancer 2017, 15, e1107–e1115. [Google Scholar] [CrossRef]
- Ryan, C.J.; Crawford, E.D.; Shore, N.D.; Underwood, W.; Taplin, M.E.; Londhe, A.; Francis, P.S.J.; Phillips, J.; McGowan, T.; Kantoff, P.W. The IMAAGEN Study: Effect of Abiraterone Acetate and Prednisone on Prostate Specific Antigen and Radiographic Disease Progression in Patients with Nonmetastatic Castration Resistant Prostate Cancer. J. Urol. 2018, 200, 344–352. [Google Scholar] [CrossRef]
- Sakamoto, S.; Maimaiti, M.; Xu, M.; Kamada, S.; Yamada, Y.; Kitoh, H.; Matsumoto, H.; Takeuchi, N.; Higuchi, K.; Uchida, H.A.; et al. Higher Serum Testosterone Levels Associated with Favorable Prognosis in Enzalutamide- and Abiraterone-Treated Castration-Resistant Prostate Cancer. J. Clin. Med. 2019, 8, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Glasgow Prognostic Score | Score |
| C-reactive protein < 1.0 mg/dL and albumin ≥ 3.5 g/dL | 0 |
| C-reactive protein ≥ 1.0 mg/dL or albumin < 3.5 g/dL | 1 |
| C-reactive protein ≥ 1.0 mg/dL and albumin < 3.5 g/dL | 2 |
| Modified Glasgow prognostic score | Score |
| C-reactive protein < 1.0 mg/dL and albumin ≥ 3.5 g/dL | 0 |
| C-reactive protein ≥ 1.0 mg/dL and albumin ≥ 3.5 g/dL | 1 |
| C-reactive protein ≥ 1.0 mg/dL and albumin < 3.5 g/dL | 2 |
| High-sensitivity modified Glasgow prognostic score | Score |
| C-reactive protein < 0.3 mg/dL and albumin ≥ 3.5 g/dL | 0 |
| C-reactive protein ≥ 0.3 mg/dL and albumin ≥ 3.5 g/dL | 1 |
| C-reactive protein ≥ 0.3 mg/dL and albumin < 3.5 g/dL | 2 |
| Characteristics | Value: Median (IQR) or n (%) |
|---|---|
| Age (years) | 72.0 (67.0–77.0) |
| BMI (kg/m2) | 23.6 (21.6–25.8) |
| Follow-up duration (months) | 21.1 (10.6–38.9) |
| ADT duration before docetaxel (months) | 35.5 (18.2–61.0) |
| Gleason score (n = 88) | |
| 5–7 | 17 (19.2%) |
| 8–10 | 59 (80.9%) |
| Metastasis | |
| Bone metastasis | 101 (80.8%) |
| Visceral metastasis | 12 (10.2%) |
| PSA (ng/mL) | 28.9 (4.6–73.4) |
| Testosterone (ng/dL) | 13.0 (5.0–23.0) |
| Lactate dehydrogenase (IU/L) | 212.0 (183.0–248.5) |
| Alkaline phosphatase (IU/L) | 295.5 (226.5–471.0) |
| Total protein (g/dL) | 6.6 (6.4–6.9) |
| Albumin (g/dL) | 4.1 (3.7–4.3) |
| Total cholesterol (mg/dL) | 216.0 (190.5–241.5) |
| Hemoglobin (g/dL) | 12.5 (11.5–13.2) |
| Platelet (×103/μL) | 2.2 (1.8–2.6) |
| C-reactive protein (mg/dL) | 0.15 (0.10–0.50) |
| Pre-ARATs | |
| No | 106 (80.9%) |
| Enzalutamide | 8 (6.1%) |
| Abiraterone | 17 (13.0%) |
| Overall Survival | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 73 year | 1.05 (0.65–1.71) | 0.8177 | ||
| BMI ≥ 23.7 kg/m2 | 1.08 (0.55–2.12) | 0.8036 | ||
| Clinical tumor stage ≥ 3 | 2.76 (0.37–20.16) | 0.3150 | ||
| Gleason score ≥ 8 | 1.20 (0.55–2.60) | 0.6419 | ||
| Visceral metastasis | 2.10 (0.88–5.01) | 0.0934 | ||
| Bone metastasis | 2.08 (1.05–4.13) | 0.0399 | 2.10 (0.78–5.60) | 0.1375 |
| PSA ≥ 27.0 ng/mL | 3.17 (1.87–5.36) | <0.0001 | 2.36 (1.23–4.54) | 0.0097 |
| Lactate dehydrogenase ≥ 213 IU/L | 1.02 (0.62–1.67) | 0.9356 | ||
| Alkaline phosphatase ≥ 293 IU/L | 1.80 (1.11–2.95) | 0.0174 | 1.13 (0.61–2.09) | 0.6880 |
| Total protein (g/dL) | 0.95 (0.49–1.83) | 0.8984 | ||
| Total cholesterol (mg/dL) | 0.96 (0.48–1.93) | 0.9297 | ||
| Testosterone ≥ 13.0 ng/dL | 2.88 (1.56–5.31) | 0.0007 | 2.23 (1.19–4.16) | 0.0017 |
| Hemoglobin (g/dL) | 0.65 (0.40–1.08) | 0.0923 | ||
| Platelet (×103/μL) | 1.35 (0.82–2.21) | 0.2277 | ||
| Hs-mGPS ≥ 1 | 2.97 (1.81–4.87) | <0.0001 | 2.41 (1.31–4.46) | 0.0048 |
| Progression-Free Survival | Univariate Analysis | Multivariate Analysis | ||
| HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 73 year | 0.73 (0.49–1.10) | 0.1396 | ||
| BMI ≥ 23.7 kg/m2 | 1.05 (0.61–1.80) | 0.8563 | ||
| Clinical tumor stage ≥ 3 | 3.06 (0.74–12.54 | 0.1198 | ||
| Gleason score ≥ 8 | 1.72 (0.87–3.41) | 0.1168 | ||
| Visceral metastasis | 1.25 (0.62–2.50) | 0.5258 | ||
| Bone metastasis | 2.34 (1.29–4.22) | 0.0047 | 1.79 (0.84–3.79) | 0.1266 |
| PSA ≥ 27.0 ng/mL | 3.80 (2.38–6.07) | <0.0001 | 2.45 (1.32–4.54) | 0.0044 |
| Lactate dehydrogenase ≥ 213 IU/L | 1.12 (0.74–1.68) | 0.5808 | ||
| Alkaline phosphatase ≥ 293 IU/L | 1.82 (1.20–2.74) | 0.0042 | 0.84 (0.47–1.51) | 0.5751 |
| Total protein (g/dL) | 0.87 (0.50–1.51) | 0.6376 | ||
| Total cholesterol (mg/dL) | 1.29 (0.72–2.31) | 0.3857 | ||
| Testosterone ≥13.0 ng/dL | 2.01 (1.23–3.25) | 0.0049 | 1.42 (0.84–2.39) | 0.1876 |
| Hemoglobin (g/dL) | 0.71 (0.47–1.07) | 0.1091 | ||
| Platelet (×103/μL) | 1.38 (0.92–2.07) | 0.1182 | ||
| Hs-mGPS ≥ 1 | 1.76 (1.16–2.68) | 0.0078 | 1.55 (0.92–2.59) | 0.0945 |
| Characteristics | Median (IQR) or n (%) | p | |
|---|---|---|---|
| Hs-mGPS < 1 | Hs-mGPS ≥ 1 | ||
| Age (years) | 72.0 (67.0–76.0) | 72.5 (67.3–77.0) | 0.7703 † |
| BMI (kg/m2) | 23.6 (22.0–25.3) | 23.7 (21.4–25.9) | 0.9698 † |
| ADT duration before docetaxel (months) | 33.1 (17.8–61.1) | 35.6 (18.5–62.0) | 0.7109 † |
| Gleason score ≥ 8 (n = 88) | 50 (83.3%) | 21 (75.0%) | 0.3642 ¶ |
| Bone metastasis | 65 (77.4%) | 36 (87.8%) | 0.1518 ¶ |
| Visceral metastasis | 8 (9.8%) | 4 (10.8%) | 0.8768 ¶ |
| PSA (ng/mL) | 21.7 (4.1–59.6) | 41.9 (14.1–125.6) | 0.0271 † |
| Testosterone (ng/dL) | 9.0 (3.0–20.5) | 18.0 (10.0–27.0) | 0.0182 † |
| Lactate dehydrogenase (IU/L) | 209.0 (179.0–273.2) | 229.0 (186.0–288.0) | 0.0260 † |
| Alkaline phosphatase (IU/L) | 286.0 (219.0–450.0) | 369.0 (240.0–685.0) | 0.0388 † |
| Hemoglobin (g/dL) | 12.7 (11.9–13.2) | 11.8 (11.0–12.9) | 0.0033 † |
| Platelet (×103/μL) | 21.6 (18.7–26.2) | 22.4 (18.0–24.9) | 0.8561 † |
| Pre-ARATs | |||
| No | 71 (80.7%) | 35 (81.4%) | 0.9221 ¶ |
| Enzalutamide | 5 (5.7%) | 3 (6.9%) | |
| Abiraterone | 12 (13.6%) | 5 (11.6%) | |
| Post-docetaxel treatment | |||
| No | 58 (65.9%) | 34 (79.0%) | 0.1149 ¶ |
| Enzalutamide | 10 (11.4%) | 6 (13.9%) | |
| Abiraterone | 11 (12.5%) | 1 (2.3%) | |
| Cabazitaxel | 9 (10.2%) | 2 (4.7%) | |
| Prognostic Factors - | Score |
|---|---|
| Hs-mGPS | |
| CRP < 0.3 mg/dL and albumin ≥ 3.5 g/dL | 0 |
| CRP ≥ 0.3 mg/dL and albumin ≥ 3.5 g/dL | 1 |
| CRP ≥ 0.3 mg/dL and albumin < 3.5 g/dL | 2 |
| PSA score | |
| PSA < 28.9 ng/mL | 0 |
| PSA ≥ 28.9 ng/mL | 1 |
| TST score | |
| TST < 13.0 ng/dL | 0 |
| TST ≥ 13.0 ng/dL | 1 |
| Risk Groups | Combined score * |
| Low | 0–1 |
| Intermediate | 2–3 |
| High | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, K.; Sakamoto, S.; Saito, S.; Maimaiti, M.; Imamura, Y.; Sazuka, T.; Sato, N.; Komiya, A.; Anzai, N.; Ichikawa, T. Prognostic Value of High-Sensitivity Modified Glasgow Prognostic Score in Castration-Resistant Prostate Cancer Patients Who Received Docetaxel. Cancers 2021, 13, 773. https://doi.org/10.3390/cancers13040773
Ando K, Sakamoto S, Saito S, Maimaiti M, Imamura Y, Sazuka T, Sato N, Komiya A, Anzai N, Ichikawa T. Prognostic Value of High-Sensitivity Modified Glasgow Prognostic Score in Castration-Resistant Prostate Cancer Patients Who Received Docetaxel. Cancers. 2021; 13(4):773. https://doi.org/10.3390/cancers13040773
Chicago/Turabian StyleAndo, Keisuke, Shinichi Sakamoto, Shinpei Saito, Maihulan Maimaiti, Yusuke Imamura, Tomokazu Sazuka, Nobuo Sato, Akira Komiya, Naohiko Anzai, and Tomohiko Ichikawa. 2021. "Prognostic Value of High-Sensitivity Modified Glasgow Prognostic Score in Castration-Resistant Prostate Cancer Patients Who Received Docetaxel" Cancers 13, no. 4: 773. https://doi.org/10.3390/cancers13040773
APA StyleAndo, K., Sakamoto, S., Saito, S., Maimaiti, M., Imamura, Y., Sazuka, T., Sato, N., Komiya, A., Anzai, N., & Ichikawa, T. (2021). Prognostic Value of High-Sensitivity Modified Glasgow Prognostic Score in Castration-Resistant Prostate Cancer Patients Who Received Docetaxel. Cancers, 13(4), 773. https://doi.org/10.3390/cancers13040773