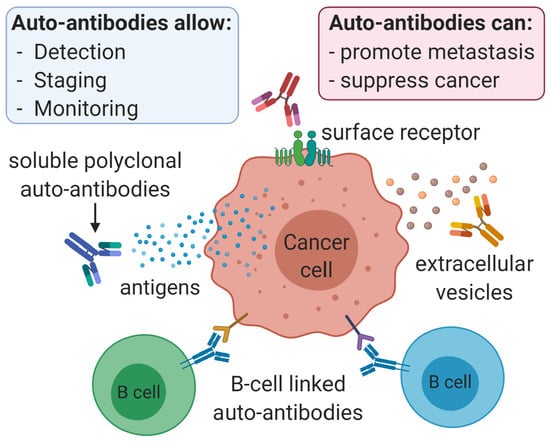
Anti-Cancer Auto-Antibodies: Roles, Applications and Open Issues
Abstract
:Simple Summary
Abstract
1. Introduction
2. Cancer Auto-Antibodies
2.1. Head and Neck Cancer
2.2. Central Nervous System Cancers
2.3. Gastrointestinal Tumors
2.3.1. Gastric Cancer
2.3.2. Colorectal Cancer
2.3.3. Pancreatic Cancer
2.3.4. Liver Cancer
2.3.5. Esophageal Cancer
2.4. Thyroid Cancer
2.5. Lung Cancer
2.6. Breast Cancer
2.7. Adrenocortical Carcinoma
2.8. Ovarian Cancer
2.9. Cervical Cancer
2.10. Bladder Cancer
2.11. Prostate Cancer
2.12. Testicular Seminoma
2.13. Lymphomas
2.14. Melanoma
2.15. Angiosarcoma
2.16. Antibody-Mediated Paraneoplastic Syndromes
3. Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Vlagea, A.; Falagan, S.; Gutierrez-Gutierrez, G.; Moreno-Rubio, J.; Merino, M.; Zambrana, F.; Casado, E.; Sereno, M. Antinuclear antibodies and cancer: A literature review. Crit. Rev. Oncol./Hematol. 2018, 127, 42–49. [Google Scholar] [CrossRef]
- Valencia, J.C.; Egbukichi, N.; Erwin-Cohen, R.A. Autoimmunity and Cancer, the Paradox Comorbidities Challenging Therapy in the Context of Preexisting Autoimmunity. J. Interf. Cytokine Res. 2019, 39, 72–84. [Google Scholar] [CrossRef]
- Bernatsky, S.; Easton, D.F.; Dunning, A.; Michailidou, K.; Ramsey-Goldman, R.; Gordon, C.; Clarke, A.E.; Foulkes, W. Decreased breast cancer risk in systemic lupus erythematosus: The search for a genetic basis continues. Lupus 2012, 21, 896–899. [Google Scholar] [CrossRef]
- Schairer, C.; Pfeiffer, R.M.; Gadalla, S.M. Autoimmune diseases and breast cancer risk by tumor hormone-receptor status among elderly women. Int. J. Cancer 2018, 142, 1202–1208. [Google Scholar] [CrossRef]
- Rosenblum, M.D.; Remedios, K.A.; Abbas, A.K. Mechanisms of human autoimmunity. J. Clin. Investig. 2015, 125, 2228–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Fahrmann, J.F.; Hanash, S.M.; Vykoukal, J. Extracellular Vesicles Mediate B Cell Immune Response and Are a Potential Target for Cancer Therapy. Cells 2020, 9. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Glotzbecker, B.; Mills, H.; Vasir, B.; Tzachanis, D.; Levine, J.D.; Joyce, R.M.; Wellenstein, K.; Keefe, W.; Schickler, M.; et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J. Immunother. 2011, 34, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordan, J.D.; Vonderheide, R.H. Universal tumor antigens as targets for immunotherapy. Cytotherapy 2002, 4, 317–327. [Google Scholar] [CrossRef]
- Suppiah, A.; Greenman, J. Clinical utility of anti-p53 auto-antibody: Systematic review and focus on colorectal cancer. World J. Gastroenterol. 2013, 19, 4651–4670. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Al-Khadairi, G.; Roelands, J.; Hendrickx, W.; Dermime, S.; Bedognetti, D.; Decock, J. NY-ESO-1 Based Immunotherapy of Cancer: Current Perspectives. Front. Immunol. 2018, 9, 947. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jia, G.; Zhao, X.; Bao, Y.; Zhang, Y.; Ozkan, C.; Minev, B.; Ma, W. Novel Survivin Peptides Screened With Computer Algorithm Induce Cytotoxic T Lymphocytes With Higher Cytotoxic Efficiency to Cancer Cells. Front. Mol. Biosci. 2020, 7, 570003. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Song, W.; Fang, Y.; Yu, L.; Liu, S.; Churilov, L.P.; Zhang, F. The roles and applications of autoantibodies in progression, diagnosis, treatment and prognosis of human malignant tumours. Autoimmun. Rev. 2017, 16, 1270–1281. [Google Scholar] [CrossRef]
- Lohmueller, J.; Finn, O.J. Current modalities in cancer immunotherapy: Immunomodulatory antibodies, CARs and vaccines. Pharmacol. Ther. 2017, 178, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Iamele, L.; Vecchia, L.; Scotti, C. Antibody–drug conjugates: Targeted weapons against cancer. Antib. Technol. J. 2015, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Affara, N.I.; Ruffell, B.; Medler, T.R.; Gunderson, A.J.; Johansson, M.; Bornstein, S.; Bergsland, E.; Steinhoff, M.; Li, Y.; Gong, Q.; et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell 2014, 25, 809–821. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Wang, Z.; Liu, C.; Xu, C.; Mbofung, R.M.; McKenzie, J.A.; Khong, H.; Hwu, P.; Peng, W. CpG-based immunotherapy impairs antitumor activity of BRAF inhibitors in a B-cell-dependent manner. Oncogene 2017, 36, 4081–4086. [Google Scholar] [CrossRef] [PubMed]
- Pucci, F.; Garris, C.; Lai, C.P.; Newton, A.; Pfirschke, C.; Engblom, C.; Alvarez, D.; Sprachman, M.; Evavold, C.; Magnuson, A.; et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 2016, 352, 242–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodt, P.; Gordon, J. Natural resistance mechanisms may play a role in protection against chemical carcinogenesis. Cancer Immunol. Immunother. 1982, 13, 125–127. [Google Scholar] [CrossRef]
- Gupta, P.; Chen, C.; Chaluvally-Raghavan, P.; Pradeep, S. B Cells as an Immune-Regulatory Signature in Ovarian Cancer. Cancers 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, R.L.; Pottala, J.V.; Nagata, S.; Egland, K.A. Longitudinal autoantibody responses against tumor-associated antigens decrease in breast cancer patients according to treatment modality. BMC Cancer 2018, 18, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, N.; Fedewa, S.; Chen, A.Y. Epidemiology and Demographics of the Head and Neck Cancer Population. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 381–395. [Google Scholar] [CrossRef]
- Hsieh, J.C.; Wang, H.M.; Wu, M.H.; Chang, K.P.; Chang, P.H.; Liao, C.T.; Liau, C.T. Review of emerging biomarkers in head and neck squamous cell carcinoma in the era of immunotherapy and targeted therapy. Head Neck 2019, 41 (Suppl. 1), 19–45. [Google Scholar] [CrossRef] [Green Version]
- Schutt, C.A.; Mirandola, L.; Figueroa, J.A.; Nguyen, D.D.; Cordero, J.; Bumm, K.; Judson, B.L.; Chiriva-Internati, M. The cancer-testis antigen, sperm protein 17, a new biomarker and immunological target in head and neck squamous cell carcinoma. Oncotarget 2017, 8, 100280–100287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordes, C.; Von Lingen, J.; Gorogh, T.; Ambrosch, P.; Gottschlich, S.; Hoffmann, M. Molecular and immunological aspects of p53 and p53-autoantibodies in head and neck squamous cell carcinoma. Oncol. Rep. 2009, 22, 1299–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaskas, N.M.; Moore-Medlin, T.; McClure, G.B.; Ekshyyan, O.; Vanchiere, J.A.; Nathan, C.A. Serum biomarkers in head and neck squamous cell cancer. JAMA Otolaryngol. Neck Surg. 2014, 140, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Nabors, L.B.; Ammirati, M.; Bierman, P.J.; Brem, H.; Butowski, N.; Chamberlain, M.C.; DeAngelis, L.M.; Fenstermaker, R.A.; Friedman, A.; Gilbert, M.R.; et al. Central nervous system cancers. J. Natl. Compr. Cancer Netw. 2013, 11, 1114–1151. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Deng, Z.; Wang, H.; Li, X.; Sun, T.; Tao, Z.; Yao, L.; Jin, Y.; Wang, X.; Yang, L.; et al. MGMT autoantibodies as a potential prediction of recurrence and treatment response biomarker for glioma patients. Cancer Med. 2019, 8, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mukherjee, S.; Syed, P.; Pandala, N.G.; Choudhary, S.; Singh, V.A.; Singh, N.; Zhu, H.; Epari, S.; Noronha, S.B.; et al. Evaluation of autoantibody signatures in meningioma patients using human proteome arrays. Oncotarget 2017, 8, 58443–58456. [Google Scholar] [CrossRef] [Green Version]
- Patti, G.; Calandra, E.; De Bellis, A.; Gallizia, A.; Crocco, M.; Napoli, F.; Allegri, A.M.E.; Thiabat, H.F.; Bellastella, G.; Maiorino, M.I.; et al. Antibodies Against Hypothalamus and Pituitary Gland in Childhood-Onset Brain Tumors and Pituitary Dysfunction. Front. Endocrinol. 2020, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, I.; Nabeya, Y.; Takiguchi, N.; Gunji, H.; Ishige, F.; Iwatate, Y.; Shiratori, F.; Yajima, S.; Okada, R.; Shimada, H. Prognostic impact of p53 and/or NY-ESO-1 autoantibody induction in patients with gastroenterological cancers. Ann. Gastroenterol. Surg. 2020, 4, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Yajima, S.; Suzuki, T.; Oshima, Y.; Shiratori, F.; Funahashi, K.; Kawai, S.; Nanki, T.; Muraoka, S.; Urita, Y.; Saida, Y.; et al. New Assay System Elecsys Anti-p53 to Detect Serum Anti-p53 Antibodies in Esophageal Cancer Patients and Colorectal Cancer Patients: Multi-institutional Study. Ann. Surg. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Chen, H.; Tao, S.; Brenner, H. Systematic review: Serum autoantibodies in the early detection of gastric cancer. Int. J. Cancer 2015, 136, 2243–2252. [Google Scholar] [CrossRef] [Green Version]
- Nanami, T.; Hoshino, I.; Ito, M.; Yajima, S.; Oshima, Y.; Suzuki, T.; Shiratori, F.; Nabeya, Y.; Funahashi, K.; Shimada, H. Prevalence of autoantibodies against Ras-like GTPases, RalA, in patients with gastric cancer. Mol. Clin. Oncol. 2020, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Suzuki, T.; Yajima, S.; Nanami, T.; Shiratori, F.; Funahashi, K.; Shimada, H. Serum p53 antibody: Useful for detecting gastric cancer but not for predicting prognosis after surgery. Surg. Today 2020, 50, 1402–1408. [Google Scholar] [CrossRef]
- Qin, J.; Wang, S.; Shi, J.; Ma, Y.; Wang, K.; Ye, H.; Zhang, X.; Wang, P.; Wang, X.; Song, C.; et al. Using recursive partitioning approach to select tumor-associated antigens in immunodiagnosis of gastric adenocarcinoma. Cancer Sci. 2019, 110, 1829–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Qin, J.; Ye, H.; Wang, K.; Shi, J.; Ma, Y.; Duan, Y.; Song, C.; Wang, X.; Dai, L.; et al. Using a panel of multiple tumor-associated antigens to enhance autoantibody detection for immunodiagnosis of gastric cancer. Oncoimmunology 2018, 7, e1452582. [Google Scholar] [CrossRef] [Green Version]
- Meistere, I.; Werner, S.; Zayakin, P.; Silina, K.; Rulle, U.; Pismennaja, A.; Santare, D.; Kikuste, I.; Isajevs, S.; Leja, M.; et al. The Prevalence of Cancer-Associated Autoantibodies in Patients with Gastric Cancer and Progressive Grades of Premalignant Lesions. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, I.; Nagata, M.; Takiguchi, N.; Nabeya, Y.; Ikeda, A.; Yokoi, S.; Kuwajima, A.; Tagawa, M.; Matsushita, K.; Satoshi, Y.; et al. Panel of autoantibodies against multiple tumor-associated antigens for detecting gastric cancer. Cancer Sci. 2017, 108, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, B.; Li, X.; Zhou, D.; Li, Y.; Jia, S.; Qi, S.; Xu, A.; Zhao, X.; Wang, J.; et al. Identification and Validation of Novel Serum Autoantibody Biomarkers for Early Detection of Colorectal Cancer and Advanced Adenoma. Front. Oncol. 2020, 10, 1081. [Google Scholar] [CrossRef]
- Liu, X.X.; Ye, H.; Wang, P.; Zhang, Y.; Zhang, J.Y. Identification of 1433zeta as a potential biomarker in gastric cancer by proteomicsbased analysis. Mol. Med. Rep. 2017, 16, 7759–7765. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Wang, S.; Wang, P.; Wang, X.; Ye, H.; Song, C.; Dai, L.; Wang, K.; Jiang, B.; Zhang, J. Autoantibody against 14-3-3 zeta: A serological marker in detection of gastric cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 1253–1262. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhou, D.; Huang, J. Autoantibodies as biomarkers for colorectal cancer: A systematic review, meta-analysis, and bioinformatics analysis. Int. J. Biol. Markers 2019, 34, 334–347. [Google Scholar] [CrossRef] [Green Version]
- Teras, L.R.; Gapstur, S.M.; Maliniak, M.L.; Jacobs, E.J.; Gansler, T.; Michel, A.; Pawlita, M.; Waterboer, T.; Campbell, P.T. Prediagnostic Antibodies to Serum p53 and Subsequent Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Ushigome, M.; Shimada, H.; Miura, Y.; Yoshida, K.; Kaneko, T.; Koda, T.; Nagashima, Y.; Suzuki, T.; Kagami, S.; Funahashi, K. Changing pattern of tumor markers in recurrent colorectal cancer patients before surgery to recurrence: Serum p53 antibodies, CA19-9 and CEA. Int. J. Clin. Oncol. 2020, 25, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Garranzo-Asensio, M.; San Segundo-Acosta, P.; Poves, C.; Fernandez-Acenero, M.J.; Martinez-Useros, J.; Montero-Calle, A.; Solis-Fernandez, G.; Sanchez-Martinez, M.; Rodriguez, N.; Ceron, M.A.; et al. Identification of tumor-associated antigens with diagnostic ability of colorectal cancer by in-depth immunomic and seroproteomic analysis. J. Proteom. 2020, 214, 103635. [Google Scholar] [CrossRef]
- Ushigome, M.; Nabeya, Y.; Soda, H.; Takiguchi, N.; Kuwajima, A.; Tagawa, M.; Matsushita, K.; Koike, J.; Funahashi, K.; Shimada, H. Multi-panel assay of serum autoantibodies in colorectal cancer. Int. J. Clin. Oncol. 2018, 23, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Patel, R.; Rehman, H.; Goyal, S.; Wasif Saif, M. Recent advances in immunotherapy for pancreatic cancer. J. Cancer Metastasis Treat. 2020, 6. [Google Scholar] [CrossRef]
- Gautam, S.K.; Kumar, S.; Dam, V.; Ghersi, D.; Jain, M.; Batra, S.K. MUCIN-4 (MUC4) is a novel tumor antigen in pancreatic cancer immunotherapy. Semin. Immunol. 2020, 47, 101391. [Google Scholar] [CrossRef]
- Hontani, K.; Tsuchikawa, T.; Hiwasa, T.; Nakamura, T.; Ueno, T.; Kushibiki, T.; Takahashi, M.; Inoko, K.; Takano, H.; Takeuchi, S.; et al. Identification of novel serum autoantibodies against EID3 in non-functional pancreatic neuroendocrine tumors. Oncotarget 2017, 8, 106206–106221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, R.; Otsuka, Y.; Wakabayashi, T.; Shinoda, M.; Aoki, T.; Murakami, M.; Arizumi, S.; Yamamoto, M.; Aramaki, O.; Takayama, T.; et al. Six autoantibodies as potential serum biomarkers of hepatocellular carcinoma: A prospective multicenter study. Int. J. Cancer 2020, 147, 2578–2586. [Google Scholar] [CrossRef]
- Welberry, C.; Macdonald, I.; McElveen, J.; Parsy-Kowalska, C.; Allen, J.; Healey, G.; Irving, W.; Murray, A.; Chapman, C. Tumor-associated autoantibodies in combination with alpha-fetoprotein for detection of early stage hepatocellular carcinoma. PLoS ONE 2020, 15, e0232247. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Qin, J.; Sun, G.; Dai, L.; Wang, P.; Ye, H.; Shi, J.; Cheng, L.; Yang, Q.; et al. Serological Biomarkers for Early Detection of Hepatocellular Carcinoma: A Focus on Autoantibodies against Tumor-Associated Antigens Encoded by Cancer Driver Genes. Cancers 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Koziol, J.A.; Imai, H.; Dai, L.; Zhang, J.Y.; Tan, E.M. Early detection of hepatocellular carcinoma using autoantibody profiles from a panel of tumor-associated antigens. Cancer Immunol. Immunother. 2018, 67, 835–841. [Google Scholar] [CrossRef]
- Ren, B.; Zou, G.; Xu, F.; Huang, Y.; Xu, G.; He, J.; Li, Y.; Zhu, H.; Yu, P. Serum levels of anti-sperm-associated antigen 9 antibody are elevated in patients with hepatocellular carcinoma. Oncol. Lett. 2017, 14, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, F.F.; Lu, M.D.; Zhang, S.X.; Li, Y.X. Anti-NY-ESO-1 autoantibody may be a tumor marker for intrahepatic cholangiocarcinoma. Oncotarget 2017, 8, 103283–103289. [Google Scholar] [CrossRef] [Green Version]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef]
- Perisetti, A.; Bellamkonda, M.; Konda, M.; Edwards, S.; Ali Khan, S.; Bansal, P.; Hu, Z.D.; Goyal, H. Tumor-associated antigens and their antibodies in the screening, diagnosis, and monitoring of esophageal cancers. Eur. J. Gastroenterol. Hepatol. 2020, 32, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Bennett, W.P.; Hollstein, M.C.; Metcalf, R.A.; Welsh, J.A.; He, A.; Zhu, S.M.; Kusters, I.; Resau, J.H.; Trump, B.F.; Lane, D.P.; et al. p53 mutation and protein accumulation during multistage human esophageal carcinogenesis. Cancer Res. 1992, 52, 6092–6097. [Google Scholar] [PubMed]
- Takashi, S.; Satoshi, Y.; Akihiko, O.; Naoya, Y.; Yusuke, T.; Kentaro, M.; Yu, O.; Yasuaki, N.; Koichi, Y.; Takashi, F.; et al. Clinical impact of preoperative serum p53 antibody titers in 1487 patients with surgically treated esophageal squamous cell carcinoma: A multi-institutional study. Esophagus 2021, 18, 65–71. [Google Scholar] [CrossRef]
- Zhang, J.B.; Cao, M.; Chen, J.; Ye, S.R.; Xie, K.; He, X.; Ma, X.L.; Zhang, J.; Yie, S.M. Serum anti-TOPO48 autoantibody as a biomarker for early diagnosis and prognosis in patients with esophageal squamous cell carcinoma. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Shimada, H.; Yajima, S.; Nanami, T.; Matsushita, K.; Nomura, F.; Kainuma, O.; Takiguchi, N.; Soda, H.; Ueda, T.; et al. NY-ESO-1 autoantibody as a tumor-specific biomarker for esophageal cancer: Screening in 1969 patients with various cancers. J. Gastroenterol. 2016, 51, 30–34. [Google Scholar] [CrossRef]
- Xu, Y.W.; Chen, H.; Guo, H.P.; Yang, S.H.; Luo, Y.H.; Liu, C.T.; Huang, X.Y.; Tang, X.M.; Hong, C.Q.; Li, E.M.; et al. Combined detection of serum autoantibodies as diagnostic biomarkers in esophagogastric junction adenocarcinoma. Gastric Cancer 2019, 22, 546–557. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xu, L.; Xiao, D.; Xie, J.; Zeng, H.; Cai, W.; Niu, Y.; Yang, Z.; Shen, Z.; Li, E. Fascin is a potential biomarker for early-stage oesophageal squamous cell carcinoma. J. Clin. Pathol. 2006, 59, 958–964. [Google Scholar] [CrossRef]
- Miao, S.; Zhou, S.Y.; Han, C.S.; Zhang, L.N.; Sun, H.B.; Yang, B. Clinicopathological significance of matrix metalloproteinase-7 protein expression in esophageal cancer: A meta-analysis. Drug Des. Dev. Ther. 2015, 9, 3729–3740. [Google Scholar] [CrossRef] [Green Version]
- Giovanella, L. Circulating biomarkers for the detection of tumor recurrence in the postsurgical follow-up of differentiated thyroid carcinoma. Curr. Opin. Oncol. 2020, 32, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ernaga-Lorea, A.; Hernandez-Morhain, M.C.; Anda-Apinaniz, E.; Pineda-Arribas, J.J.; Migueliz-Bermejo, I.; Eguilaz-Esparza, N.; Irigaray-Echarri, A. Prognostic value of change in anti-thyroglobulin antibodies after thyroidectomy in patients with papillary thyroid carcinoma. Clin. Transl. Oncol. 2018, 20, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Reverter, J.L.; Rosas-Allende, I.; Puig-Jove, C.; Zafon, C.; Megia, A.; Castells, I.; Pizarro, E.; Puig-Domingo, M.; Granada, M.L. Prognostic Significance of Thyroglobulin Antibodies in Differentiated Thyroid Cancer. J. Thyroid. Res. 2020, 2020, 8312628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P.; et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J. Thorac. Oncol. 2019, 14, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.; Boyle, P.; Healey, G.F.; Maddison, P.; Peek, L.; Murray, A.; Chapman, C.J.; Allen, J.; Wood, W.C.; Sewell, H.F.; et al. EarlyCDT-Lung: An immunobiomarker test as an aid to early detection of lung cancer. Cancer Prev. Res. 2011, 4, 1126–1134. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, I.K.; Murray, A.; Healey, G.F.; Parsy-Kowalska, C.B.; Allen, J.; McElveen, J.; Robertson, C.; Sewell, H.F.; Chapman, C.J.; Robertson, J.F. Application of a high throughput method of biomarker discovery to improvement of the EarlyCDT((R))-Lung Test. PLoS ONE 2012, 7, e51002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, C.J.; Healey, G.F.; Murray, A.; Boyle, P.; Robertson, C.; Peek, L.J.; Allen, J.; Thorpe, A.J.; Hamilton-Fairley, G.; Parsy-Kowalska, C.B.; et al. EarlyCDT(R)-Lung test: Improved clinical utility through additional autoantibody assays. Tumor Biol. 2012, 33, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Healey, G.F.; Lam, S.; Boyle, P.; Hamilton-Fairley, G.; Peek, L.J.; Robertson, J.F. Signal stratification of autoantibody levels in serum samples and its application to the early detection of lung cancer. J. Thorac. Dis. 2013, 5, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Massion, P.P.; Healey, G.F.; Peek, L.J.; Fredericks, L.; Sewell, H.F.; Murray, A.; Robertson, J.F. Autoantibody Signature Enhances the Positive Predictive Power of Computed Tomography and Nodule-Based Risk Models for Detection of Lung Cancer. J. Thorac. Oncol. 2017, 12, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Edelsberg, J.; Weycker, D.; Atwood, M.; Hamilton-Fairley, G.; Jett, J.R. Cost-effectiveness of an autoantibody test (EarlyCDT-Lung) as an aid to early diagnosis of lung cancer in patients with incidentally detected pulmonary nodules. PLoS ONE 2018, 13, e0197826. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, F.M.; Farmer, E.; Mair, F.S.; Treweek, S.; Kendrick, D.; Jackson, C.; Robertson, C.; Briggs, A.; McCowan, C.; Bedford, L.; et al. Detection in blood of autoantibodies to tumour antigens as a case-finding method in lung cancer using the EarlyCDT(R)-Lung Test (ECLS): Study protocol for a randomized controlled trial. BMC Cancer 2017, 17, 187. [Google Scholar] [CrossRef] [Green Version]
- Jett, J.R.; Peek, L.J.; Fredericks, L.; Jewell, W.; Pingleton, W.W.; Robertson, J.F. Audit of the autoantibody test, EarlyCDT(R)-lung, in 1600 patients: An evaluation of its performance in routine clinical practice. Lung Cancer 2014, 83, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, P.; Chapman, C.J.; Holdenrieder, S.; Murray, A.; Robertson, C.; Wood, W.C.; Maddison, P.; Healey, G.; Fairley, G.H.; Barnes, A.C.; et al. Clinical validation of an autoantibody test for lung cancer. Ann. Oncol. 2011, 22, 383–389. [Google Scholar] [CrossRef]
- Pei, L.; Liu, H.; Ouyang, S.; Zhao, C.; Liu, M.; Wang, T.; Wang, P.; Ye, H.; Wang, K.; Song, C.; et al. Discovering novel lung cancer associated antigens and the utilization of their autoantibodies in detection of lung cancer. Immunobiology 2020, 225, 151891. [Google Scholar] [CrossRef]
- Pan, J.; Yu, L.; Wu, Q.; Lin, X.; Liu, S.; Hu, S.; Rosa, C.; Eichinger, D.; Pino, I.; Zhu, H.; et al. Integration of IgA and IgG Autoantigens Improves Performance of Biomarker Panels for Early Diagnosis of Lung Cancer. Mol. Cell. Proteom. 2020, 19, 490–500. [Google Scholar] [CrossRef]
- Yang, B.; Ren, N.; Guo, B.; Xin, H.; Yin, Y. Measuring serum human epididymis secretory protein autoantibody as an early biomarker of lung cancer. Transl. Cancer Res. 2019, 9, 735–741. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, N.; Wang, H.; Kobayashi, M.; Ladd, J.J.; Long, J.P.; Lo, K.C.; Patel, J.; Sullivan, E.; Albert, T.; et al. Whole Genome-Derived Tiled Peptide Arrays Detect Prediagnostic Autoantibody Signatures in Non-Small-Cell Lung Cancer. Cancer Res. 2019, 79, 1549–1557. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Zhang, S.; Jiang, T.; He, Y.; Ma, Z.; Cai, H.; Xu, X.; Li, Y.; Cai, W.; Zhou, J.; et al. Early detection of lung cancer by using an autoantibody panel in Chinese population. Oncoimmunology 2018, 7, e1384108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Ma, L.; Li, W.; Zhou, S.; Xu, S. Diagnostic value of multiple tumor-associated autoantibodies in lung cancer. Onco Targets Ther. 2019, 12, 457–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Zhang, X.; Jiang, Q.; Liang, T. Detection of circulating natural antibodies against CD25, MUC1, and VEGFR1 for early diagnosis of non-small cell lung cancer. FEBS Open Bio 2020, 10, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Zang, R.; Li, Y.; Jin, R.; Wang, X.; Lei, Y.; Che, Y.; Lu, Z.; Mao, S.; Huang, J.; Liu, C.; et al. Enhancement of diagnostic performance in lung cancers by combining CEA and CA125 with autoantibodies detection. Oncoimmunology 2019, 8, e1625689. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, Y.; Liu, M.; Si, Q.; Wang, T.; Pei, L.; Wang, P.; Ye, H.; Shi, J.; Wang, X.; et al. A panel of autoantibodies against multiple tumor-associated antigens in the early immunodiagnosis of lung cancer. Immunobiology 2020, 225, 151848. [Google Scholar] [CrossRef] [PubMed]
- Giannicola, R.; D’Arrigo, G.; Botta, C.; Agostino, R.; Del Medico, P.; Falzea, A.C.; Barbieri, V.; Staropoli, N.; Del Giudice, T.; Pastina, P.; et al. Early blood rise in auto-antibodies to nuclear and smooth muscle antigens is predictive of prolonged survival and autoimmunity in metastatic-non-small cell lung cancer patients treated with PD-1 immune-check point blockade by nivolumab. Mol. Clin. Oncol. 2019, 11, 81–90. [Google Scholar] [CrossRef]
- Djureinovic, D.; Hallstrom, B.M.; Horie, M.; Mattsson, J.S.M.; La Fleur, L.; Fagerberg, L.; Brunnstrom, H.; Lindskog, C.; Madjar, K.; Rahnenfuhrer, J.; et al. Profiling cancer testis antigens in non-small-cell lung cancer. JCI Insight 2016, 1, e86837. [Google Scholar] [CrossRef] [Green Version]
- Djureinovic, D.; Dodig-Crnkovic, T.; Hellstrom, C.; Holgersson, G.; Bergqvist, M.; Mattsson, J.S.M.; Ponten, F.; Stahle, E.; Schwenk, J.M.; Micke, P. Detection of autoantibodies against cancer-testis antigens in non-small cell lung cancer. Lung Cancer 2018, 125, 157–163. [Google Scholar] [CrossRef]
- Ait-Tahar, K.; Damm-Welk, C.; Burkhardt, B.; Zimmermann, M.; Klapper, W.; Reiter, A.; Pulford, K.; Woessmann, W. Correlation of the autoantibody response to the ALK oncoantigen in pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma with tumor dissemination and relapse risk. Blood 2010, 115, 3314–3319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, M.M.; Mastini, C.; Blasco, R.B.; Mologni, L.; Voena, C.; Mussolin, L.; Mach, S.L.; Adeni, A.E.; Lydon, C.A.; Sholl, L.M.; et al. Epitope mapping of spontaneous autoantibodies to anaplastic lymphoma kinase (ALK) in non-small cell lung cancer. Oncotarget 2017, 8, 92265–92274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Keyser, B.; Lin, Z.T.; Wu, T. Autoantibodies as Potential Biomarkers in Breast Cancer. Biosensors 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmetz, C.; Bibeau, F.; Boissiere, F.; Bellet, V.; Rouanet, P.; Maudelonde, T.; Mange, A.; Solassol, J. Proteomics-based identification of HSP60 as a tumor-associated antigen in early stage breast cancer and ductal carcinoma in situ. J. Proteome Res. 2008, 7, 3830–3837. [Google Scholar] [CrossRef]
- Katayama, H.; Boldt, C.; Ladd, J.J.; Johnson, M.M.; Chao, T.; Capello, M.; Suo, J.; Mao, J.; Manson, J.E.; Prentice, R.; et al. An Autoimmune Response Signature Associated with the Development of Triple-Negative Breast Cancer Reflects Disease Pathogenesis. Cancer Res. 2015, 75, 3246–3254. [Google Scholar] [CrossRef] [Green Version]
- Qiu, C.; Wang, P.; Wang, B.; Shi, J.; Wang, X.; Li, T.; Qin, J.; Dai, L.; Ye, H.; Zhang, J. Establishment and validation of an immunodiagnostic model for prediction of breast cancer. Oncoimmunology 2020, 9, 1682382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aras, S.; Maroun, M.C.; Song, Y.; Bandyopadhyay, S.; Stark, A.; Yang, Z.Q.; Long, M.P.; Grossman, L.I.; Fernandez-Madrid, F. Mitochondrial autoimmunity and MNRR1 in breast carcinogenesis. BMC Cancer 2019, 19, 411. [Google Scholar] [CrossRef]
- Tokunaga, E.; Takizawa, K.; Masuda, T.; Ijichi, H.; Koga, C.; Tajiri, W.; Tanaka, J.; Nakamura, Y.; Taguchi, K.; Ishida, M. Tumor-infiltrating lymphocytes and serum anti-p53 autoantibody in HER2-positive breast cancer treated with neoadjuvant chemotherapy. J. Clin. Oncol. 2018, 36, e12648. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, Y.; Fu, L.; Zhai, L.; Zhu, J.; Han, Y.; Jiang, Y.; Zhang, Y.; Zhang, P.; Jiang, Z.; et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat. Med. 2019, 25, 312–322. [Google Scholar] [CrossRef]
- Doghman-Bouguerra, M.; Finetti, P.; Durand, N.; Parise, I.Z.S.; Sbiera, S.; Cantini, G.; Canu, L.; Hescot, S.; Figueiredo, M.M.O.; Komechen, H.; et al. Cancer-testis Antigen FATE1 Expression in Adrenocortical Tumors Is Associated with A Pervasive Autoimmune Response and Is A Marker of Malignancy in Adult, but Not Children, ACC. Cancers 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- Fortner, R.T.; Schock, H.; Le Cornet, C.; Husing, A.; Vitonis, A.F.; Johnson, T.S.; Fichorova, R.N.; Fashemi, T.; Yamamoto, H.S.; Tjonneland, A.; et al. Ovarian cancer early detection by circulating CA125 in the context of anti-CA125 autoantibody levels: Results from the EPIC cohort. Int. J. Cancer 2018, 142, 1355–1360. [Google Scholar] [CrossRef]
- Kobayashi, M.; Katayama, H.; Irajizad, E.; Vykoukal, J.V.; Fahrmann, J.F.; Kundnani, D.L.; Yu, C.Y.; Cai, Y.; Hsiao, F.C.; Yang, W.L.; et al. Proteome Profiling Uncovers an Autoimmune Response Signature That Reflects Ovarian Cancer Pathogenesis. Cancers 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.L.; Gentry-Maharaj, A.; Simmons, A.; Ryan, A.; Fourkala, E.O.; Lu, Z.; Baggerly, K.A.; Zhao, Y.; Lu, K.H.; Bowtell, D.; et al. Elevation of TP53 Autoantibody Before CA125 in Preclinical Invasive Epithelial Ovarian Cancer. Clin. Cancer Res. 2017, 23, 5912–5922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katchman, B.A.; Chowell, D.; Wallstrom, G.; Vitonis, A.F.; LaBaer, J.; Cramer, D.W.; Anderson, K.S. Autoantibody biomarkers for the detection of serous ovarian cancer. Gynecol. Oncol. 2017, 146, 129–136. [Google Scholar] [CrossRef]
- Hurley, L.C.; Levin, N.K.; Chatterjee, M.; Coles, J.; Muszkat, S.; Howarth, Z.; Dyson, G.; Tainsky, M.A. Evaluation of paraneoplastic antigens reveals TRIM21 autoantibodies as biomarker for early detection of ovarian cancer in combination with autoantibodies to NY-ESO-1 and TP53. Cancer Biomark. 2020, 27, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Antony, F.; Deantonio, C.; Cotella, D.; Soluri, M.F.; Tarasiuk, O.; Raspagliesi, F.; Adorni, F.; Piazza, S.; Ciani, Y.; Santoro, C.; et al. High-throughput assessment of the antibody profile in ovarian cancer ascitic fluids. Oncoimmunology 2019, 8, e1614856. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Li, L. Identification of six serum antigens and autoantibodies for the detection of early stage epithelial ovarian carcinoma by bioinformatics analysis and liquid chip analysis. Oncol. Lett. 2018, 16, 3231–3240. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.T.; Scanlan, M.J.; Sahin, U.; Tureci, O.; Gure, A.O.; Tsang, S.; Williamson, B.; Stockert, E.; Pfreundschuh, M.; Old, L.J. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc. Natl. Acad. Sci. USA 1997, 94, 1914–1918. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.L.; Moffitt, L.R.; Duffield, N.; Rainczuk, A.; Jobling, T.W.; Plebanski, M.; Stephens, A.N. Autoantibodies against HSF1 and CCDC155 as Biomarkers of Early-Stage, High-Grade Serous Ovarian Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortner, R.T.; Damms-Machado, A.; Kaaks, R. Systematic review: Tumor-associated antigen autoantibodies and ovarian cancer early detection. Gynecol. Oncol. 2017, 147, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Van Hoesen, K.; Meynier, S.; Ribaux, P.; Petignat, P.; Delie, F.; Cohen, M. Circulating GRP78 antibodies from ovarian cancer patients: A promising tool for cancer cell targeting drug delivery system? Oncotarget 2017, 8, 107176–107187. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Kim, S.C.; Kim, H.J.; Ju, W.; Kim, Y.H.; Kim, H.J. Use of autoantibodies against tumor-associated antigens as serum biomarkers for primary screening of cervical cancer. Oncotarget 2017, 8, 105425–105439. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.L.; Kim, H.J.; Kim, S.C.; Ju, W.; Kim, Y.H.; Chang, K.H.; Kim, H.J. Serum anti-GAPDH autoantibody levels reflect the severity of cervical lesions: A potential serum biomarker for cervical cancer screening. Oncol. Lett. 2019, 18, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Richters, A.; Aben, K.K.H.; Kiemeney, L. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [Green Version]
- Isharwal, S.; Konety, B. Non-muscle invasive bladder cancer risk stratification. Indian J. Urol. 2015, 31, 289–296. [Google Scholar] [CrossRef]
- Minami, S.; Matsumoto, K.; Nagashio, R.; Hagiuda, D.; Fukuda, E.; Goshima, N.; Hattori, M.; Tsuchiya, B.; Hachimura, K.; Jiang, S.X.; et al. Analysis of Autoantibodies Related to Tumor Progression in Sera from Patients with High-grade Non-muscle-invasive Bladder Cancer. Anticancer Res. 2017, 37, 6705–6714. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wan, L.; Zhang, J.; Zhang, J.; Mendez, L.; Clohessy, J.G.; Berry, K.; Victor, J.; Yin, Q.; Zhu, Y.; et al. Deregulated PP1alpha phosphatase activity towards MAPK activation is antagonized by a tumor suppressive failsafe mechanism. Nat. Commun. 2018, 9, 159. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.-H.; Martinez, A.; Rastogi, A.; Huang, W.; Banerjee, S.; Ravindranath, L.; Young, D.; Ali, A.; Kohaar, I.; Chen, Y.; et al. Abstract 3296: Tumor Antigens Fetuin-A and Secreted Protein Acidic and Rich in Cysteine (SPARC) Autoantibodies as Diagnostic and Prognostic Biomarkers in Prostate Cancer; America Association for Cancer Research: Philadelphia, PA, USA, 2019; p. 3296. [Google Scholar]
- Voltz, R.; Gultekin, S.H.; Rosenfeld, M.R.; Gerstner, E.; Eichen, J.; Posner, J.B.; Dalmau, J. A serologic marker of paraneoplastic limbic and brain-stem encephalitis in patients with testicular cancer. N. Engl. J. Med. 1999, 340, 1788–1795. [Google Scholar] [CrossRef]
- Graus, F.; Delattre, J.Y.; Antoine, J.C.; Dalmau, J.; Giometto, B.; Grisold, W.; Honnorat, J.; Smitt, P.S.; Vedeler, C.; Verschuuren, J.J.; et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1135–1140. [Google Scholar] [CrossRef] [Green Version]
- Pruss, H.; Voltz, R.; Flath, B.; Rudolph, B.; Klingebiel, R.; Zschenderlein, R.; Prass, K. Anti-Ta-associated paraneoplastic encephalitis with occult testicular intratubular germ-cell neoplasia. J. Neurol. Neurosurg. Psychiatry 2007, 78, 651–652. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, L.A.; Jarius, S.; Pellkofer, H.L.; Schueller, M.; Krumbholz, M.; Koenig, F.; Johannis, W.; la Fougere, C.; Newman, T.; Vincent, A.; et al. Anti-Ma and anti-Ta associated paraneoplastic neurological syndromes: 22 newly diagnosed patients and review of previous cases. J. Neurol. Neurosurg. Psychiatry 2008, 79, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Mandel-Brehm, C.; Dubey, D.; Kryzer, T.J.; O’Donovan, B.D.; Tran, B.; Vazquez, S.E.; Sample, H.A.; Zorn, K.C.; Khan, L.M.; Bledsoe, I.O.; et al. Kelch-like Protein 11 Antibodies in Seminoma-Associated Paraneoplastic Encephalitis. N. Engl. J. Med. 2019, 381, 47–54. [Google Scholar] [CrossRef]
- Maudes, E.; Landa, J.; Munoz-Lopetegi, A.; Armangue, T.; Alba, M.; Saiz, A.; Graus, F.; Dalmau, J.; Sabater, L. Clinical significance of Kelch-like protein 11 antibodies. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Bilici, A.; Yapici, H.S.; Ercan, S.; Seker, M.; Ustaalioglu, B.B.; Salman, T.; Orcun, A.; Gumus, M. The prevalence and significance of autoantibodies in patients with non-Hodgkin’s lymphoma: Are they correlated with clinicopathological features? J. BUON 2012, 17, 502–507. [Google Scholar]
- Swissa, M.; Cohen, Y.; Shoenfeld, Y. Autoantibodies in the sera of patients with lymphoma. Leuk. Lymphoma 1992, 7, 117–122. [Google Scholar] [CrossRef]
- Khodadoust, M.S.; Olsson, N.; Wagar, L.E.; Haabeth, O.A.; Chen, B.; Swaminathan, K.; Rawson, K.; Liu, C.L.; Steiner, D.; Lund, P.; et al. Antigen presentation profiling reveals recognition of lymphoma immunoglobulin neoantigens. Nature 2017, 543, 723–727. [Google Scholar] [CrossRef] [Green Version]
- Svane, I.M.; Verdegaal, E.M. Achievements and challenges of adoptive T cell therapy with tumor-infiltrating or blood-derived lymphocytes for metastatic melanoma: What is needed to achieve standard of care? Cancer Immunol. Immunother. 2014, 63, 1081–1091. [Google Scholar] [CrossRef]
- Lauss, M.; Donia, M.; Harbst, K.; Andersen, R.; Mitra, S.; Rosengren, F.; Salim, M.; Vallon-Christersson, J.; Torngren, T.; Kvist, A.; et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat. Commun. 2017, 8, 1738. [Google Scholar] [CrossRef] [Green Version]
- Snyder, A.; Wolchok, J.D.; Chan, T.A. Genetic basis for clinical response to CTLA-4 blockade. N. Engl. J. Med. 2015, 372, 783. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaenker, P.; Lo, J.; Pearce, R.; Cantwell, P.; Cowell, L.; Lee, M.; Quirk, C.; Law, H.; Gray, E.; Ziman, M. A diagnostic autoantibody signature for primary cutaneous melanoma. Oncotarget 2018, 9, 30539–30551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penel, N.; Marreaud, S.; Robin, Y.M.; Hohenberger, P. Angiosarcoma: State of the art and perspectives. Crit. Rev. Oncol./Hematol. 2011, 80, 257–263. [Google Scholar] [CrossRef]
- Kiyohara, M.; Aoi, J.; Kajihara, I.; Otuka, S.; Kadomatsu, T.; Fukushima, S.; Ihn, H. Serum anti-p53 autoantibodies in angiosarcoma. J. Dermatol. 2020, 47, 849–854. [Google Scholar] [CrossRef]
- Inuzuka, T. Paraneoplastic neurological syndrome—Definition and history. Brain Nerve. 2010, 62, 301–308. [Google Scholar] [PubMed]
- Takkar, A.; Mehta, S.; Gupta, N.; Bansal, S.; Lal, V. Anti- RI antibody associated progressive supranuclear palsy like presentation in a patient with breast carcinoma. J. Neuroimmunol. 2020, 347, 577345. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A. Antiphospholipid antibodies and antiphospholipid syndrome in cancer: Uninvited guests in troubled times. Semin. Cancer Biol. 2020, 64, 108–113. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Tayar, J.H.; Fa’ak, F.; Sharma, G.; Lopez-Olivo, M.A.; Yousif, A.; Shagroni, T.; Al-Hawamdeh, S.; Rojas-Hernandez, C.M.; Suarez-Almazor, M.E. Systematic review of observational studies reporting antiphospholipid antibodies in patients with solid tumors. Blood Adv. 2020, 4, 1746–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prevezianou, A.; Tzartos, J.S.; Dagklis, I.E.; Bentenidi, E.; Angelopoulos, P.; Bostantjopoulou, S. Paraneoplastic cerebellar degeneration in a patient with breast cancer associated with carbonic anhydrase-related protein VIII autoantibodies. J. Neuroimmunol. 2020, 344, 577242. [Google Scholar] [CrossRef]
- Yshii, L.; Bost, C.; Liblau, R. Immunological Bases of Paraneoplastic Cerebellar Degeneration and Therapeutic Implications. Front. Immunol. 2020, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Lionaki, S.; Marinaki, S.; Panagiotellis, K.; Tsoumbou, I.; Liapis, G.; Vlahadami, I.; Tzioufas, A.; Sfikakis, P.; Boletis, I. Glomerular Diseases Associated with Malignancies: Histopathological Pattern and Association with Circulating Autoantibodies. Antibodies 2020, 9. [Google Scholar] [CrossRef]
- Zekeridou, A.; Majed, M.; Heliopoulos, I.; Lennon, V.A. Paraneoplastic autoimmunity and small-cell lung cancer: Neurological and serological accompaniments. Thorac. Cancer 2019, 10, 1001–1004. [Google Scholar] [CrossRef] [Green Version]
- Borgese, N.; Colombo, S.; Pedrazzini, E. The tale of tail-anchored proteins: Coming from the cytosol and looking for a membrane. J. Cell Biol. 2003, 161, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Syed, P.; Gupta, S.; Choudhary, S.; Pandala, N.G.; Atak, A.; Richharia, A.; Kp, M.; Zhu, H.; Epari, S.; Noronha, S.B.; et al. Autoantibody Profiling of Glioma Serum Samples to Identify Biomarkers Using Human Proteome Arrays. Sci. Rep. 2015, 5, 13895. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Ye, H.; Wang, X.; Li, T.; Jiang, D.; Qiu, C.; Dai, L.; Shi, J.; Wang, K.; Song, C.; et al. Autoantibodies against tumor-associated antigens combined with microRNAs in detecting esophageal squamous cell carcinoma. Cancer Med. 2020, 9, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- The Relations of Cancer to Chronic Inflammation. Hospital 1909, 46, 349–350.
- Burman, L.; Chong, Y.E.; Duncan, S.; Klaus, A.; Rauch, K.; Hamel, K.; Herve, K.; Pfaffen, S.; Collins, D.W.; Heyries, K.; et al. Isolation of monoclonal antibodies from anti-synthetase syndrome patients and affinity maturation by recombination of independent somatic variants. MAbs 2020, 12, 1836718. [Google Scholar] [CrossRef]
| Antigen (Short Name/Gene Name) | Antigen Name | Uniprot ID | Cancer | I/E *,§ | Sensitivity | Specificity | Notes |
|---|---|---|---|---|---|---|---|
| 14-3-3ζ | 14-3-3 protein zeta | P29310 | Gastric cancer | I | 22.58% | 92.26% | |
| ACTR3 | Actin-related protein 3 | P61158 | Lung cancer | I | 20.7% | >90% | Early stage marker |
| AEG-1 | Protein Lyric | Q86UE4 | Gastrointestinal cancer | I | 59.1% | 100% | Stage-related (late-stage patients) |
| AHSG | Alpha-2-HS-glycoprotein (Fetuin-A) | P02765 | Prostate cancer | E | N.A. | N.A. | |
| ALDH1B1 | Aldehyde dehydrogenase X | P30837 | Colorectal cancer | I | 62.31–75.68% | 73.78–63.06% | |
| ALK | Anaplastic lymphoma kinase | Q9UM73 | Lung cancer | E | N.A. | N.A. | NSCLC inversely correlated with stage of disease |
| ALMS1 | Alstrom syndrome protein 1 | Q8TCU4 | Lung cancer | I | N.A. | N.A. | |
| Annexin-1 | Annexin A1 | P04083 | Lung cancer | E | N.A. | N.A. | |
| Colorectal cancer | 2.5% | N.A. | |||||
| ATP1A4 | Sodium/potassium-transporting ATPase subunit alpha-4 | Q13733 | Lung cancer | E | N.A. | N.A. | |
| BCL7A | B cell CLL/lymphoma 7 protein family member A | Q4VC05 | Lung cancer | N.A. | 30.9% | 94.3% | IgA autoantigen, early stage marker |
| BCOR | BCL-6 corepressor | Q6W2J9 | Ovarian cancer | I | 73% | >94% | |
| C1D | Nuclear nucleic acid-binding protein C1D | Q13901 | Ovarian cancer | I | N.A. | N.A. | |
| C12orf54 | Uncharacterized protein | Q6X4T0 | Lung cancer | N.A. | N.A. | N.A. | |
| CA125 | Mucin-16 (Cancer Antigen 125) | Q8WXI7 | Lung cancer | E | N.A. | N.A. | |
| Ovarian cancer | 95% | 40% | |||||
| CA19-9 | Tetrasaccharide CA19-9 | N.A. | Cervical cancer | N.A. | 3.2% | N.A. | |
| CAGE | Cancer-associated gene 1 protein | Q8TC20 | Lung cancer | N.A. | N.A. | N.A. | |
| CAMSAP2 | Calmodulin-regulated spectrin-associated protein 2 | Q08AD1 | Lung cancer | I | N.A. | N.A. | |
| CCDC155 | Coiled-coil domain-containing protein 155 | Q8N6L0 | Ovarian cancer | I | 95% | 40% | Early ovarian cancer |
| CCL18 | C-C motif chemokine 18 | P55774 | Ovarian cancer | E | N.A. | N.A. | |
| CCNB1 | G2/mitotic-specific cyclin-B1 | P14635 | Central nervous system (CNS) tumors | I | 10.6% | 96.2% | |
| CD25 | Interleukin-2 receptor subunit alpha | P01589 | Lung cancer | E | N.A. | N.A. | |
| CEA | Carcinoembryonic antigen-related cell adhesion molecule 5 | P06731 | Lung cancer | E | N.A. | N.A. | (Antigen only) |
| CENPF | Centromere protein F | P49454 | Colorectal cancer | I | 64.34% | 67.27% | Colorectal cancer |
| 62.67% | 62.67% | Advanced adenoma | |||||
| CREB3 | Cyclic AMP-responsive element-binding protein 3 | O43889 | Ovarian cancer | I | 87% | 98% | |
| CRYM | Ketimine reductase mu-crystallin | Q14894 | Central nervous system (CNS) tumors | I | N.A. | N.A. | Downregulated in menangiomas |
| CT47A | Cancer/testis antigen 47A | Q5JQC4 | Lung cancer | N.A. | N.A. | N.A. | |
| CTAG1 | Cancer/testis antigen 1 | P78358 | Gastric cancer | I | N.A. | N.A. | |
| Colorectal cancer | 64.62–59.46% | 70.27–56.36% | Colorectal cancer, advanced adenoma | ||||
| Lung cancer | 23.5% | 97.7% | IgG autoantigen, early stage marker | ||||
| CTAG2 | Cancer/testis antigen 2 | O75638 | Gastrointestinal cancer | I | 16.6% | 99.5% | Not stage-related |
| CXCL1 | Growth-regulated alpha protein | P09341 | Ovarian cancer | E | N.A. | N.A. | |
| Cyclin B1 | G2/mitotic-specific cyclin-B1 | P14635 | Colorectal cancer | I | 15.6–32.7% | 97.6% | Hepatocarcinoma |
| Cytokeratin 20 | Keratin, type I cytoskeletal 20 | P35900 | Lung cancer | I | N.A. | N.A. | |
| DDX4 | Probable ATP-dependent RNA helicase DDX4 | Q9NQI0 | Lung cancer | I | 25.0% | 96.6% | IgG autoantigenearly-stage marker |
| DDX53 | Probable ATP-dependent RNA helicase DDX53 | Q86TM3 | Gastrointestinal cancer | I | 6.8% | 100% | Not stage-related |
| EFCAB2 | Dynein regulatory complex protein 8 | Q5VUJ9 | Central nervous system (CNS) tumors | I | N.A. | N.A. | |
| ENO1 | Alpha-enolase | P06733 | Gastric cancer | I | N.A. | N.A. | |
| ERP44 | Endoplasmic reticulum resident protein 44 | Q9BS26 | Colorectal cancer | I | 40% | 100% | |
| FSCN1 | Fascin | Q16658 | Esophageal cancer | I | N.A. | N.A. | |
| FATE1 | Fetal and adult testis-expressed transcript protein | Q969F0 | Adrenocortical carcinoma | I | N.A. | N.A. | |
| FXR1 | Fragile X mental retardation syndrome-related protein 1 | P51114 | Ovarian cancer | I | N.A. | N.A. | |
| GAGE7 | G antigen 7 | O76087 | Lung cancer | N.A. | N.A. | N.A. | |
| Galectin1 | Galectin1 | P09382 | Colorectal cancer | E | 11% | N.A. | |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | P16858 | Cervical cancer | I | 80% | 96.6% | |
| GBU4-5 | Putative ATP-dependent RNA helicase TDRD12 | Q587J7 | Lung cancer | I | N.A. | N.A. | Also known as FLI3072 |
| GCC2 | GRIP and coiled-coil domain-containing protein 2 | Q8IWJ2 | Lung cancer | I | N.A. | N.A. | |
| GNA11 | Guanine nucleotide-binding protein subunit alpha-11 | P29992 | Liver cancer | I | N.A. | N.A. | Hepatocarcinoma |
| GNAS | Guanine nucleotide-binding protein G(s) subunit alpha isoforms short | P63092 | Liver cancer | E | 48.4% | >90% | Hepatocarcinoma |
| GPR78 | G-protein coupled receptor 78 | Q96P69 | Gastrointestinal cancer | E | 28.3% | 100% | Non stage-related |
| GREM1 | Gremlin-1 | O60565 | Lung cancer | E | N.A. | N.A. | |
| GRP78 | Glucose regulated protein 78 | P11021 | Ovarian cancer | I | N.A. | N.A. | |
| HCA25a | Hepatocellular carcinoma-associated antigen HCA25a | Q8NHH4 | Colorectal cancer | N.A. | 3% | N.A. | |
| HCC-22-5 (SMP30) | Senescence marker protein-30 (Regucalcin) | Q15493 | Colorectal cancer | I | 4% | N.A. | |
| HCC1 | Protein SCO1 homolog 1 | Q8VYP0 | Liver cancer | I | N.A. | N.A. | Hepatocarcinoma |
| HDAC7A | Histone deacetylase 7 | Q8WUI4 | CNS tumors | I | N.A. | N.A. | |
| HE4 | Human epididymis secretory protein 4 | Q14508 | Lung cancer | E | 67.21% | 96.23% | |
| HMGB3 | High mobility group protein B3 | O15347 | Lung cancer | I | N.A. | N.A. | |
| HOXA7 | Homeobox protein Hox-A7 | P31268 | Ovarian cancer | I | 66.7% | 100% | |
| HRNR | Hornerin | Q86YZ3 | Lung cancer | I | N.A. | N.A. | |
| HSF1 | Heat shock factor protein 1 | Q00613 | Ovarian cancer | I | 95% | 80% | Early ovarian cancer |
| Hspa4 | Heat shock 70 kDa protein 4 | P34932 | Breast cancer | I | N.A. | N.A. | |
| Hsp40 | DnaJ homolog subfamily B member 1 | P25685 | Colorectal cancer | I | 7% | N.A. | |
| Hsp60 | Heat shock protein 60 | P10809 | Breast cancer | I | N.A. | N.A. | 31% cases of early breast cancer, 32.6% ductal carcinoma in situ |
| Lung cancer | N.A. | N.A. | |||||
| Hsp70 | Heat shock 70 kDa protein | P0DMV8 | Colorectal cancer | I | 12% | N.A. | |
| Esophageal cancer | N.A. | N.A. | |||||
| HuD * | ELAV-like protein 4 | P26378 | Lung cancer | I | N.A. | N.A. | |
| IGF2BP2 | Insulin-like growth factor 2 mRNA-binding protein 2 | Q9Y6M1 | Gastric cancer | I | N.A. | N.A. | |
| IGHG4 | Immunoglobulin heavy constant gamma 4 | P01861 | Central nervous system (CNS) tumors | E | N.A. | N.A. | |
| IL8 | Interleukin-8 | P10145 | Ovarian cancer | E | 65.5% | 98% | Stage I–II |
| IMP1 | Insulin-like growth factor 2 mRNA-binding protein 1 | Q9NZI8 | Colorectal cancer | I | 13.3–21.7% | 97.6–100% | |
| Kelch-like protein 11 * | Kelch-like protein 11 | Q9NVR0 | Testicular seminoma | I | N.A. | N.A. | Patients with paraneoplastic encephalitis; associated with poor response to treatment |
| KIF13B | Kinesin-like protein | Q9NQT8 | Lung cancer | I | N.A. | N.A. | |
| KM-HN-1 | Coiled-coil domain-containing protein 110 | Q8TBZ0 | Colorectal cancer | I | 9% | N.A. | |
| Koc | Insulin-like growth factor 2 mRNA-binding protein 3 | O00425 | Colorectal cancer | I | 8.9–15.2% | 98.8–100% | |
| LIN28B | Protein lin-28 homolog B | Q6ZN17 | Lung cancer | I | N.A. | N.A. | |
| Lmyc2 | Protein L-Myc | P12524 | Lung cancer | I | N.A. | N.A. | |
| Ma * | Paraneoplastic antigen Ma1 | Q8ND90 | Testicular seminoma | I | N.A. | N.A. | |
| Paraneoplastic antigen Ma2 | Q9UL42 | Testicular seminoma | I | N.A. | N.A. | ||
| MAGEA1 | Melanoma-associated antigen 1 | P43355 | Lung cancer | I | N.A. | N.A. | |
| MAGEA4 | Melanoma-associated antigen 4 | P43358 | Lung cancer | N.A. | N.A. | N.A. | |
| MAGEA3 | Melanoma-associated antigen 3 | P43357 | Gastrointestinal cancer | I | 3.4% | 100% | Not stage-related |
| MAGEB1 | Melanoma-associated antigen B1 | P43366 | Lung canceer | N.A. | N.A. | N.A. | |
| MAGEC1 | Melanoma-associated antigen C1 | O60732 | Gastrointestinal cancer | N.A. | 3.4% | 100% | Not stage-related |
| MAGEC2 | Melanoma-associated antigen C2 | Q9UBF1 | Lung cancer | I | 27.9% | 95.4% | IgG autoantigen, early stage marker |
| MDM2 | E3 ubiquitin-protein ligase | Q00987 | Gastric cancer | I | N.A. | N.A. | |
| MED14 | Mediator of RNA polymerase II transcription subunit | O60244 | Lung cancer | I | N.A. | N.A. | |
| MGMT | Methylated DNA protein cysteine methyltransferase | P16455 | Central nervous system (CNS) tumors | I | N.A. | N.A. | Associated with higher risk of chemotherapy resistance and disease recurrence; positive rate: MGMT-02 45%, MGMT-04 27%, MGMT-07 21%, MGMT-10 13%, MGMT-18 24% |
| MMP-7 | Matrilysin | P09237 | Esophageal cancer | E | N.A. | N.A. | |
| MNRR1 (CHCHD2) | Mitochondrial-nuclear retrograde regulator 1 | Q9Y6H1 | Breast cancer | I | N.A. | N.A. | Metastasis and aggressive tumors |
| MPB-1 | Alpha-enolase | P06733 | Lung cancer | I | N.A. | N.A. | |
| MRPL46 | 39S ribosomal protein L46, mitochondrial | Q9H2W6 | Ovarian cancer | I | 73% | >94% | |
| MSH2 | DNA mismatch repair protein Msh2 | P43246 | Liver cancer | I | 42.1% | >90% | Hepatocarcinoma |
| MTERF4 | Transcription termination factor 4 | Q7Z6M4 | Lung cancer | I | 33.5% | 96.6% | IgA autoantigen, early stage marker |
| MUC1 | Mucin 1 | P15941 | Lung cancer | E | N.A. | N.A | Mucin 1 subunit ß is translocated in the nucleus |
| MUC17 | Mucn-17 | Q685J3 | Lung cancer | I/E | N.A. | N.A. | |
| Myc | Myc proto-oncogene protein | P01106 | Gastric cancer | I | 33.17% | 90.17% | |
| Colorectal cancer | 4.4–21.7% | 94.8–100% | |||||
| Liver cancer | 9% | N.A. | |||||
| Ovarian cancer | N.A. | N.A. | |||||
| NPM1 | Nucleophosmin | P06748 | Gastric cancer | I | N.A. | N.A. | |
| NUDT11 | Diphosphoinositol polyphosphate phosphohydrolase 3-beta | Q96G61 | Ovarian cancer | I | 32.4% | 100% | Serous ovarian cancer |
| NY-ESO-1 * | Cancer/testis antigen 1 | P78358 | Liver cancer | I | 10% | N.A. | Hepatocarcinoma Intrahepatic cholangio-carcinoma |
| Lung cancer | N.A. | N.A. | |||||
| Colorectal | 8% | N.A. | |||||
| Esophageal cancer | 31% | N.A. | |||||
| p16 | Cyclin-dependent kinase inhibitor 2A | P42771 | Esophageal cancer | I | N.A. | N.A. | |
| Gastric cancer | N.A. | N.A. | |||||
| Liver cancer | N.A. | N.A. | |||||
| p53 | Cellular tumor antigen p53 | P04637 | Breast cancer | I | N.A. | N.A. | Triple negative |
| Gastric cancer | N.A. | N.A. | In combination with CEA and/or CA19-9, associated with lymphatic node and distant metastasis | ||||
| Colorectal cancer | 16% | N.A. | |||||
| Liver cancer | 11% | N.A. | Hepatocarcinoma | ||||
| Lung cancer | N.A. | N.A. | EarlyCTD Lung panel, non-small cell lung cancer (NSCLC) | ||||
| Angiosarcoma | N.A. | N.A. | |||||
| Ovarian cancer | N.A. | N.A. | |||||
| p62 | Sequestosome-1 | Q13501 | Gastric cancer | I | N.A. | N.A. | |
| Colorectal cancer | 11.1–23.4% | 97.1–98.8% | |||||
| Liver cancer | 18% | N.A. | Hepatocarcinoma | ||||
| p90 | N.A. | G8IFA7 | Colorectal cancer | N.A. | 7% | N.A. | |
| Liver cancer | N.A. | N.A. | N.A. | Hepatocarcinoma | |||
| PAGE3 | P antigen family member 3 | Q5JUK9 | Lung cancer | N.A. | N.A. | N.A. | |
| PAX5 | Paired box protein Pax-5 | Q02548 | Liver cancer | I | N.A. | N.A. | Hepatocarcinoma |
| PGP9.5 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | P09936 | Lung cancer | I | N.A. | N.A. | |
| PI3K | Phosphatidylinositol 4,5-bisphosphate 3-kinase | P42336 | Breast cancer | I | N.A. | N.A. | Triple negative |
| PPP1C | Serine/threonine-protein phosphatase PP1-alpha catalytic subunit | P62136 | Bladder cancer | I | 64.2% | 65.7% | |
| PrxVI | Peroxiredoxin-6 | P30041 | Colorectal cancer | I | 4% | N.A. | |
| PSIP1 | PC4 and SFRS1-interacting protein | O75475 | Lung cancer | I | 32.07% | >90% | Early stage marker |
| PTCH1 | Protein patched homolog 1 | Q13635 | Liver cancer | E | N.A. | N.A. | Hepatocarcinoma |
| PTEN | Phosphatidyl-inositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase | P60484 | Gastric cancer | I/E | N.A. | N.A. | |
| PVR | Poliovirus receptor | P15151 | Ovarian cancer | E | 17.6% | 96.8% | Serous ovarian cancer |
| RalA | Ras-related protein Ral-A | P11233 | Gastric cancer | E | 15% | N.A. | In combination with CEA and/or CA19-9 |
| Colorectal cancer | 14% | N.A. | |||||
| Liver cancer | 17% | N.A. | Hepatocarcinoma | ||||
| RhoGDI | Rho GDP-dissociation inhibitor 1 | P52565 | Ovarian cancer | I | 89.5% | 80% | Ovarian cancer |
| RPS6KA5 | Ribosomal protein S6 kinase alpha-5 | O75582 | Lung cancer | I | 15.4% | >90% | Early stage marker |
| SMG1 | Serine/threonine-protein kinase | Q96Q15 | Lung cancer | I | N.A. | N.A. | |
| SOX2 | Transcription factor SOX-2 | P48431 | Lung cancer | I | N.A. | N.A. | EarlyCTD Lung panel, non-small cell lung cancer (NSCLC) |
| SP17 | Sperm surface protein Sp17 | Q15506 | Head and neck cancers (HCN) | E | N.A. | N.A. | |
| SPAG9 | Sperm-associated antigen | O60271 | Liver cancer | I | 71.0% | 87.3% | Hepatocarcinoma |
| SPARC | SPARC | P09486 | Prostate cancer | E | N.A. | N.A. | |
| STAT6 | Signal transducer and activator of transcription 6 | P42226 | CNS tumors | I | N.A. | N.A. | |
| Sui1 | Eukaryotic translation initiation factor 1 | P41567 | Liver cancer | I | 19% | N.A. | Hepatocarcinoma |
| Survivin | Baculoviral IAP repeat-containing protein 5 | O15392 | Colorectal cancer | I | 4.4–56.9% | 64.1–100% | |
| Liver cancer | 42.4% | >90% | Hepatocarcinoma | ||||
| Ta proteins * | Tail-anchored proteins | N/A | Testicular seminoma | E | N.A. | N.A. | [145] |
| TALDO1 | Transaldolase | P37837 | Colorectal cancer | I | 56.9% | 66.7% | |
| Tg | Thyroglobulin | P01266 | Differentiated thyroid carcinoma (DTC) | E | N.A. | N.A. | |
| TIMELESS | Protein timeless homolog | Q9UNS1 | Lung cancer | I | N.A. | N.A. | |
| TIZ (ZNF675) | Zinc finger protein 675 | Q8TD23 | Ovarian cancer | I | N.A. | N.A. | |
| TM4SF1 | Transmembrane 4 L6 family member 1 | P30408 | Ovarian cancer | E | N.A. | N.A. | |
| TNS1 | Tensin-1 | Q9HBL0 | Lung cancer | I | N.A. | N.A. | |
| TOP1 | DNA topoisomerase 1 fragment | P11387 | Esophageal cancer | I | 61.8% | 100% | TOP1 fragment (329–765) |
| TOP2A | DNA topoisomerase 2-alpha | P11388 | Lung cancer | I | 24.5% | >90% | Early stage marker |
| TRIM21 * | E3 ubiquitin-protein ligase TRIM21 | P19474 | Ovarian cancer | I | 33% | 100% | |
| TRIM33 * | E3 ubiquitin-protein ligase TRIM33 | Q9UPN9 | Lung cancer | I | 32.4% | 94.3% | IgA autoantigen early stage marker |
| TRIM39 | E3 ubiquitin-protein ligase TRIM39 | Q9HCM9 | Ovarian cancer | I | 14.7% | 96.8% | Serous ovarian cancer |
| TUBA1C | Tubulin alpha-1C chain | Q9BQE3 | Ovarian cancer | I | 89% | 75% | |
| UQCRC1 | Cytochrome b-c1 complex subunit 1 | P31930 | Colorectal cancer | I | 57.7% | 70.27% | Colorectal cancer |
| 86.49% | 27.93% | Advanced adenoma | |||||
| VCX | Variable charge X-linked protein 1 | Q9H320 | Lung cancer | I | N.A. | N.A. | |
| VEGF | Vascular endothelial growth factor | P15692 | Colorectal concer | E | 4% | N.A. | |
| VEGFR1 | Vascular endothelial growth factor receptor 1 | P17948 | Lung cancer | E | N.A. | N.A. |
| Panel (Antigens) | Cancer | Sensitivity | Specificity | Reference |
|---|---|---|---|---|
| c-Myc, p16, HSPD1, PTEN, p53, NPM1, ENO1, p62, HCC1.4 | Gastric cancer | 71.5% | 71.3% | [36] |
| p53, heat shock protein 70, HCC-22-5, peroxiredoxin VI, KM-HN-1, p90 TAA | Gastric cancer | 49% | 92.4% | [38] |
| 14-3-3 zeta, CEA, CA199, CA724 | Gastric cancer | 52.7% | N.A. | [41] |
| p62, c-Myc, NPM1, 14-3-3ξ, MDM2, p16 | Gastric cancer | 78.92% | 74.7 | [36] |
| CTAG1B/CTAG2, DDX53, IGF2BP2, p53, MAGEA3 | Gastric cancer | 21% | 91% | [37] |
| RalA, CEA, CA19-9 | Gastric cancer | N.A. | N.A. | [33] |
| p53, CEA, CA19-9 | Gastric cancer | N.A. | N.A. | [33] |
| c-MYC, cyclin B1, p62, Koc, IMP1, survivin | Colorectal cancer | 15.5–88% | 71.4–100% | [42] |
| ALDH1B1, UQCRC1, CTAG1, CENPF | Colorectal cancer | 75.68% | 73.87% | [39] |
| p53, RalA, HSP70, Galectin1, KM-HN-1 | Colorectal cancer | 56% | 85% | [46] |
| p53, RalA, HSP70, Galectin1, KM-HN-1, NY-ESO-1, p90, Sui1, HSP40, Cyclin B1, HCC-22-5, c-myc, PrxVI, VEGF, HCA25a, p62, Annexin | Colorectal cancer | N.A. | N.A. | [46] |
| SNX1, MYLK, VDAC1, IGHG1, CCDC32, EYA1, CD44, NOL3, PQBP1, EXOSC7 | Glioma | 88% | 87% | [146] |
| C14orf80, GCK, HSD17B14, LYPLAL1, MAGEA4, MLX, RTN4, SNX1, TEX264, ARHGAP17 | Glioma | 89% | 100% | [146] |
| PGM2, DR1, HIBADH, PGM2, DR1, HIBADH | Glioma | 77% | 95% | [146] |
| Sui1, p62, RalA, p53, NY-ESO-1, c-myc | Liver cancer | 56% | 91% | [50] |
| PTCH1, GNA11, PAX5, GNAS, MSH2, Survivin, p53 | Liver cancer | N.A. | N.A. | [53] |
| HCC1, P16, P53, P90, Survivin | Liver cancer | 88% | 84.1% | [54] |
| p53, NY-ESO-1, CAGE, GBU4–5, Annexin 1, SOX2 | Lung cancer(early CTD-lung) | 39% | 89% | [73] |
| CD25, MUC1, VEGFR1 | Lung cancer | 49.6% | 95% | [86] |
| p53, NY-ESO-1, CAGE, GBU4–5, Annexin 1, SOX2, Lmyc2 and cytokeratin 20 or alpha-enolase, cytokeratin 20 | Lung cancer (implemented early CTD-lung) | 41.6% | 92.1% | [72] |
| p53, NY-ESO-1, CAGE, GBU4-5, SOX2, HuD, MAGE A4 | Lung cancer | 41% | 91% | [73] |
| P53, SOX2, GAGE 7 | Non-small cell lung cancer | 39% | 81.8% | [85] |
| SOX2, GAGE 7, CAGE, MAGE A1, P53, GBU4-5, PGP9.5 | Non-small cell lung cancer | 90% | 57.9% | [85] |
| p53, PGP9.5, SOX2, GAGE7, GBU4-5, MAGE A1, CAGE | Lung adenocarcinoma with ground-glass nodules (GGNs) | 48.6% | 92.7% | [84] |
| BCL7A, TRIM33, MTERF4, CTAG1A, DDX4, MAGEC2 | Lung cancer | 73.5% | >85% | [81] |
| CEA, CA125, Annexin A1-Ab, Alpha enolase-Ab | Lung cancer | 86.5% | 82.3% | [87] |
| GREM1, HMGB3, PSIP1 | Lung cancer | 58.07% | 76.71% | [88] |
| MUC17, CAMSAP2, KIF13B, SMG1, MED14, ALMS1, GCC2, TIMELESS, TNS1, ATP1A4, HRNR | Lung cancer | N.A. | N.A. | [83] |
| Anti-pituitary (APA), anti-hypothalamus (AHA) | Brain cancer | N.A. | N.A. | [29] |
| p53, NY-ESO-1, MMP-7, Hsp70, PRDX6, Bmi-1 | Esophagogastric junction adenocarcinoma | 61.4% | 90% | [64] |
| HCCR, C-myc, MDM2, miR-21, miR-223, miR-375 | Esophageal squamous cell carcinoma | 69% | 90% | [147] |
| TRIM21, NY-ESO-1, p53, PAX8 | Ovarian | 46% | 98% | [106] |
| C1D, TM4SF, TIZ, FXR1 combined with CCL18 and CXCL1 antigens | Ovarian | 73.14% | 94.95% | [108] |
| ICAM3, CTAG2, p53, STYXL1, PVR, POMC, NUDT11, TRIM39, UHMK1, KSR1, NXF3 | Ovarian | 45% | 98% | [111] |
| CA15-3, CEA, CA19-9 | Cervical cancer | 90.3% | 82.1% | [113] |
| ZBTB7B, PRKCH, p53, PCTK1, PQBP1, UBE2V1, IRF4, MAPK8_tv2, MSN, TPM1 | Melanoma | 79% | 84% | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jonge, H.; Iamele, L.; Maggi, M.; Pessino, G.; Scotti, C. Anti-Cancer Auto-Antibodies: Roles, Applications and Open Issues. Cancers 2021, 13, 813. https://doi.org/10.3390/cancers13040813
de Jonge H, Iamele L, Maggi M, Pessino G, Scotti C. Anti-Cancer Auto-Antibodies: Roles, Applications and Open Issues. Cancers. 2021; 13(4):813. https://doi.org/10.3390/cancers13040813
Chicago/Turabian Stylede Jonge, Hugo, Luisa Iamele, Maristella Maggi, Greta Pessino, and Claudia Scotti. 2021. "Anti-Cancer Auto-Antibodies: Roles, Applications and Open Issues" Cancers 13, no. 4: 813. https://doi.org/10.3390/cancers13040813
APA Stylede Jonge, H., Iamele, L., Maggi, M., Pessino, G., & Scotti, C. (2021). Anti-Cancer Auto-Antibodies: Roles, Applications and Open Issues. Cancers, 13(4), 813. https://doi.org/10.3390/cancers13040813