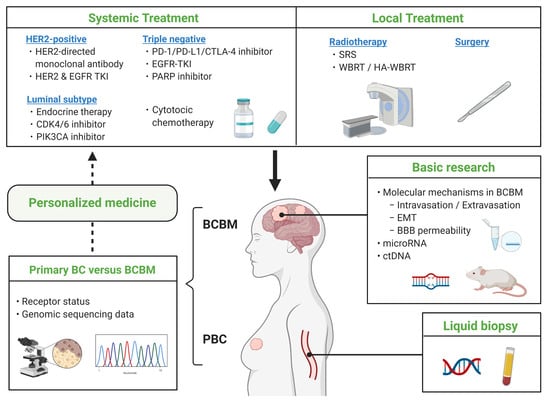
Breast Cancer Brain Metastasis—Overview of Disease State, Treatment Options and Future Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
2. Local Treatment for Patients with BCBM
2.1. Patients with Single or Two to Four BMs
2.1.1. Surgery Plus WBRT
2.1.2. Postoperative SRS vs. WBRT
2.1.3. WBRT Alone versus WBRT Plus SRS
2.1.4. SRS Only versus WBRT Plus SRS
2.2. Patients with Five or More BMs
2.3. Preoperative SRS vs. Postoperative SRS
2.4. Hippocampal-Avoidance WBRT (HA-WBRT) for BMs
3. Systemic Therapy for BCBM
3.1. Systemic Therapy for HER2-Positive BCBM
3.1.1. Trastuzumab for BCBM
3.1.2. Lapatinib for BCBM
3.1.3. Pertuzumab for BCBM
3.1.4. Trastuzumab Emtansine (T-DM1) for BCBM
3.1.5. Neratinib for BCBM
3.1.6. Tucatinib for BCBM
3.1.7. Trastuzumab Deruxtecan for BCBM
3.2. Systemic Therapy for Luminal-Type BCBM
3.2.1. Endocrine Therapy
3.2.2. PI3K Inhibitor for BCBM
3.2.3. CDK4/6 Inhibitors for BCBM
3.3. Systemic Therapy for Triple-Negative BCBM
3.3.1. Immune Checkpoint Inhibitors for BCBM
3.3.2. Bevacizumab for BCBM
3.4. New Targeted Agents for BCBM
PARP Inhibitors for BCBM
4. Receptor Status/Genomic Profiling Differences between PBC and BCBM
5. Role of Long Noncoding RNA with BCBM
6. The Association between BCBM and miRNA Expression
7. The Relationship between the BRCA1/2 Mutations and BCBM
8. Clinical Utility of Liquid Biopsy for BCBM
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Davis, F.G.; Vigneau, F.D.; Lai, P.; Sawaya, R.E. Incidence Proportions of Brain Metastases in Patients Diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004, 22, 2865–2872. [Google Scholar] [CrossRef]
- Weil, R.J.; Palmieri, D.C.; Bronder, J.L.; Stark, A.M.; Steeg, P.S. Breast Cancer Metastasis to the Central Nervous System. Am. J. Pathol. 2005, 167, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, E.M.; Shim, B.; Goodman, S.; Amonkar, M.M. Epidemiology and economic burden of brain metastases among patients with primary breast cancer: Results from a US claims data analysis. Breast Cancer Res. Treat. 2008, 108, 297–305. [Google Scholar] [CrossRef]
- Martin, A.M.; Cagney, D.N.; Catalano, P.J.; Warren, L.E.; Bellon, J.R.; Punglia, R.S.; Claus, E.B.; Lee, E.Q.; Wen, P.Y.; Haas-Kogan, D.A.; et al. Brain Metastases in Newly Diagnosed Breast Cancer. JAMA Oncol. 2017, 3, 1069. [Google Scholar] [CrossRef]
- Heitz, F.; Rochon, J.; Harter, P.; Lueck, H.J.; Fisseler-Eckhoff, A.; Barinoff, J.; Traut, A.; Lorenz-Salehi, F.; Du Bois, A. Cerebral metastases in metastatic breast cancer: Disease-specific risk factors and survival. Ann. Oncol. 2011, 22, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Tsuta, K.; Shimizu, C.; Hatanaka, Y.; Hashizume, K.; Ono, M.; Nakanishi, Y.; Hasegawa, T.; Miyakita, Y.; Narita, Y.; et al. Immunohistochemical profiles of brain metastases from breast cancer. J. Neurooncol. 2008, 90, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.M.; Mayer, M.; Rugo, H.S.; Kaufman, P.A.; Tan-Chiu, E.; Tripathy, D.; Tudor, I.C.; Wang, L.I.; Brammer, M.G.; Shing, M.; et al. Central Nervous System Metastases in Patients with HER2-Positive Metastatic Breast Cancer: Incidence, Treatment, and Survival in Patients from registHER. Clin. Cancer Res. 2011, 17, 4834–4843. [Google Scholar] [CrossRef] [Green Version]
- Ono, M.; Ando, M.; Yunokawa, M.; Nakano, E.; Yonemori, K.; Matsumoto, K.; Kouno, T.; Shimizu, C.; Tamura, K.; Katsumata, N.; et al. Brain metastases in patients who receive trastuzumab-containing chemotherapy for HER2-overexpressing metastatic breast cancer. Int. J. Clin. Oncol. 2009, 14, 48–52. [Google Scholar] [CrossRef]
- Berghoff, A.; Bago-Horvath, Z.; De Vries, C.; Dubsky, P.; Pluschnig, U.; Rudas, M.; Rottenfusser, A.; Knauer, M.; Eiter, H.; Fitzal, F.; et al. Brain metastases free survival differs between breast cancer subtypes. Br. J. Cancer 2012, 106, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Saraf, A.; Grubb, C.S.; Hwang, M.E.; Tai, C.-H.; Wu, C.-C.; Jani, A.; Lapa, M.E.; Andrews, J.I.S.; Vanderkelen, S.; Isaacson, S.R.; et al. Breast cancer subtype and stage are prognostic of time from breast cancer diagnosis to brain metastasis development. J. Neurooncol. 2017, 134, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.U.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic Behavior of Breast Cancer Subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef]
- Koniali, L.; Hadjisavvas, A.; Constantinidou, A.; Christodoulou, K.; Christou, Y.; Demetriou, C.; Panayides, A.S.; Pitris, C.; Pattichis, C.S.; Zamba-Papanicolaou, E.; et al. Risk factors for breast cancer brain metastases: A systematic review. Oncotarget 2020, 11, 650–669. [Google Scholar] [CrossRef] [Green Version]
- Rudat, V.; El-Sweilmeen, H.; Brune-Erber, I.; Nour, A.A.; Almasri, N.; Altuwaijri, S.; Fadel, E. Identification of breast cancer patients with a high risk of developing brain metastases: A single-institutional retrospective analysis. BMC Cancer 2014, 14, 289. [Google Scholar] [CrossRef] [Green Version]
- Graesslin, O.; Abdulkarim, B.S.; Coutant, C.; Huguet, F.; Gabos, Z.; Hsu, L.; Marpeau, O.; Uzan, S.; Pusztai, L.; Strom, E.A.; et al. Nomogram to Predict Subsequent Brain Metastasis in Patients With Metastatic Breast Cancer. J. Clin. Oncol. 2010, 28, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Tonyali, O.; Coskun, U.; Yuksel, S.; Inanc, M.; Bal, O.; Akman, T.; Yazilitas, D.; Ulas, A.; Kucukoner, M.; Aksoy, A.; et al. Risk factors for brain metastasis as a first site of disease recurrence in patients with HER2 positive early stage breast cancer treated with adjuvant trastuzumab. Breast 2016, 25, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.A.; Abdel-Malek, R.; Kassem, L. Predicting Brain Metastasis in Breast Cancer Patients: Stage Versus Biology. Clin. Breast Cancer 2018, 18, e187–e195. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.; Suen, D.; Ma, K.K.; Kwong, A. Identifying risk factors for brain metastasis in breast cancer patients: Implication for a vigorous surveillance program. Asian. J. Surg. 2015, 38, 220–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darlix, A.; Louvel, G.; Fraisse, J.; Jacot, W.; Brain, E.; Debled, M.; Mouret-Reynier, M.A.; Goncalves, A.; Dalenc, F.; Delaloge, S.; et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br. J. Cancer 2019, 121, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Niikura, N.; Hayashi, N.; Masuda, N.; Takashima, S.; Nakamura, R.; Watanabe, K.-I.; Kanbayashi, C.; Ishida, M.; Hozumi, Y.; Tsuneizumi, M.; et al. Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: A multicenter retrospective analysis. Breast Cancer Res. Treat. 2014, 147, 103–112. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Effect of Tumor Subtype on Survival and the Graded Prognostic Assessment for Patients With Breast Cancer and Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 2111–2117. [Google Scholar] [CrossRef] [Green Version]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary Report on the Graded Prognostic Assessment: An Accurate and Facile Diagnosis-Specific Tool to Estimate Survival for Patients With Brain Metastases. J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griguolo, G.; Jacot, W.; Kantelhardt, E.; Dieci, M.V.; Bourgier, C.; Thomssen, C.; Bailleux, C.; Miglietta, F.; Braccini, A.-L.; Conte, P.; et al. External validation of Modified Breast Graded Prognostic Assessment for breast cancer patients with brain metastases: A multicentric European experience. Breast 2018, 37, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeken, J.F.; Löscher, W. The Blood-Brain Barrier and Cancer: Transporters, Treatment, and Trojan Horses. Clin. Cancer Res. 2007, 13, 1663–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, C.W. Permeability of the Blood–Brain Barrier: Molecular Mechanism of Transport of Drugs and Physiologically Important Compounds. J. Membr. Biol. 2015, 248, 651–669. [Google Scholar] [CrossRef]
- Soffietti, R.; Ahluwalia, M.; Lin, N.; Rudà, R. Management of brain metastases according to molecular subtypes. Nat. Rev. Neurol. 2020, 16, 557–574. [Google Scholar] [CrossRef]
- Wilhelm, I.; Molnár, J.; Fazakas, C.; Haskó, J.; Krizbai, I. Role of the Blood-Brain Barrier in the Formation of Brain Metastases. Int. J. Mol. Sci. 2013, 14, 1383–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, E.R.; Fine, R.L. Increased Permeability of the Blood-Brain Barrier to Chemotherapy in Metastatic Brain Tumors: Establishing a Treatment Paradigm. J. Clin. Oncol. 2007, 25, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Bailleux, C.; Eberst, L.; Bachelot, T. Treatment strategies for breast cancer brain metastases. Br. J. Cancer 2021, 124, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous Blood–Tumor Barrier Permeability Determines Drug Efficacy in Experimental Brain Metastases of Breast Cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef] [Green Version]
- Gril, B.; Paranjape, A.N.; Woditschka, S.; Hua, E.; Dolan, E.L.; Hanson, J.; Wu, X.; Kloc, W.; Izycka-Swieszewska, E.; Duchnowska, R.; et al. Reactive astrocytic S1P3 signaling modulates the blood–tumor barrier in brain metastases. Nat. Commun. 2018, 9, s41467–s42018. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Nishimura, M.C.; Lacap, J.A.; Kharbanda, S.; Mai, E.; Tien, J.; Malesky, K.; Williams, S.P.; Marik, J.; Phillips, H.S. Trastuzumab uptake and its relation to efficacy in an animal model of HER2-positive breast cancer brain metastasis. Breast Cancer Res. Treat. 2017, 164, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabraji, S.; Ni, J.; Lin, N.U.; Xie, S.; Winer, E.P.; Zhao, J.J. Drug Resistance in HER2-Positive Breast Cancer Brain Metastases: Blame the Barrier or the Brain? Clin. Cancer Res. 2018, 24, 1795–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; De Jong, J.R.; Van Dongen, G.A.; Schröder, C.P.; Lub-De Hooge, M.N.; De Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET Imaging of HER2-Positive Lesions in Patients With Metastatic Breast Cancer. Clin. Pharm. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Taskar, K.S.; Rudraraju, V.; Mittapalli, R.K.; Samala, R.; Thorsheim, H.R.; Lockman, J.; Gril, B.; Hua, E.; Palmieri, D.; Polli, J.W.; et al. Lapatinib Distribution in HER2 Overexpressing Experimental Brain Metastases of Breast Cancer. Pharm. Res. 2012, 29, 770–781. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, A.; Peereboom, D.M.; Thorsheim, H.R.; Samala, R.; Balyan, R.; Murphy, C.G.; Lockman, P.R.; Simmons, A.; Weil, R.J.; Tabar, V.; et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: A prospective study. Neuro Oncol. 2015, 17, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Tsao, M.N.; Rades, D.; Wirth, A.; Lo, S.S.; Danielson, B.L.; Gaspar, L.E.; Sperduto, P.W.; Vogelbaum, M.A.; Radawski, J.D.; Wang, J.Z.; et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pr. Radiat. Oncol. 2012, 2, 210–225. [Google Scholar] [CrossRef] [Green Version]
- Nabors, L.B.; Portnow, J.; Ahluwalia, M.; Baehring, J.; Brem, H.; Brem, S.; Butowski, N.; Campian, J.L.; Clark, S.W.; Fabiano, A.J.; et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 1537–1570. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A Randomized Trial of Surgery in the Treatment of Single Metastases to the Brain. N. Engl. J. Med. 1990, 322, 494–500. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative Radiotherapy in the Treatment of Single Metastases to the Brain. JAMA 1998, 280. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Kayama, T.; Sato, S.; Sakurada, K.; Mizusawa, J.; Nishikawa, R.; Narita, Y.; Sumi, M.; Miyakita, Y.; Kumabe, T.; Sonoda, Y.; et al. Effects of Surgery with Salvage Stereotactic Radiosurgery Versus Surgery with Whole-Brain Radiation Therapy in Patients with One to Four Brain Metastases (JCOG0504): A Phase III, Noninferiority, Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 3282–3289. [Google Scholar] [CrossRef]
- Andrews, D.W.; Scott, C.B.; Sperduto, P.W.; Flanders, A.E.; Gaspar, L.E.; Schell, M.C.; Werner-Wasik, M.; Demas, W.; Ryu, J.; Bahary, J.P.; et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet 2004, 363, 1665–1672. [Google Scholar] [CrossRef]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villà, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. Adjuvant Whole-Brain Radiotherapy Versus Observation after Radiosurgery or Surgical Resection of One to Three Cerebral Metastases: Results of the EORTC 22952-26001 Study. J. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery with Whole Brain Radiation Therapy on Cognitive Function in Patients with 1 to 3 Brain Metastases. JAMA 2016, 316, 401. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic Radiosurgery Plus Whole-Brain Radiation Therapy vs. Stereotactic Radiosurgery Alone for Treatment of Brain Metastases. JAMA 2006, 295, 2483. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Li, J.; Ludmir, E.B.; Wang, Y.; Guha-Thakurta, N.; McAleer, M.F.; Settle, S.H.; Yeboa, D.N.; Ghia, A.J.; McGovern, S.L.; Chung, C.; et al. Stereotactic Radiosurgery versus Whole-brain Radiation Therapy for Patients with 4-15 Brain Metastases: A Phase III Randomized Controlled Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, S21–S22. [Google Scholar] [CrossRef]
- Gondi, V.; Pugh, S.L.; Tome, W.A.; Caine, C.; Corn, B.; Kanner, A.; Rowley, H.; Kundapur, V.; Denittis, A.; Greenspoon, J.N.; et al. Preservation of Memory with Conformal Avoidance of the Hippocampal Neural Stem-Cell Compartment during Whole-Brain Radiotherapy for Brain Metastases (RTOG 0933): A Phase II Multi-Institutional Trial. J. Clin. Oncol. 2014, 32, 3810–3816. [Google Scholar] [CrossRef]
- Ewend, M.G.; Morris, D.E.; Carey, L.A.; Ladha, A.M.; Brem, S. Guidelines for the Initial Management of Metastatic Brain Tumors: Role of Surgery, Radiosurgery, and Radiation Therapy. J. Natl. Compr. Cancer Netw. 2008, 6, 505–514. [Google Scholar] [CrossRef]
- Vecht, C.J.; Haaxma-Reiche, H.; Noordijk, E.M.; Padberg, G.W.; Voormolen, J.H.; Hoekstra, F.H.; Tans, J.T.; Lambooij, N.; Metsaars, J.A.; Wattendorff, A.R.; et al. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann. Neurol. 1993, 33, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Mintz, A.H.; Kestle, J.; Rathbone, M.P.; Gaspar, L.; Hugenholtz, H.; Fisher, B.; Duncan, G.; Skingley, P.; Foster, G.; Levine, M. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996, 78, 1470–1476. [Google Scholar] [CrossRef]
- Churilla, T.M.; Chowdhury, I.H.; Handorf, E.; Collette, L.; Collette, S.; Dong, Y.; Alexander, B.M.; Kocher, M.; Soffietti, R.; Claus, E.B.; et al. Comparison of Local Control of Brain Metastases with Stereotactic Radiosurgery vs Surgical Resection. JAMA Oncol. 2019, 5, 243. [Google Scholar] [CrossRef] [PubMed]
- Kępka, L.; Tyc-Szczepaniak, D.; Bujko, K.; Olszyna-Serementa, M.; Michalski, W.; Sprawka, A.; Trąbska-Kluch, B.; Komosińska, K.; Wasilewska-Teśluk, E.; Czeremszyńska, B. Stereotactic radiotherapy of the tumor bed compared to whole brain radiotherapy after surgery of single brain metastasis: Results from a randomized trial. Radiother. Oncol. 2016, 121, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Patel, A.; Lunsford, L.D.; Kassam, A.; Flickinger, J.C. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 427–434. [Google Scholar] [CrossRef]
- Tsao, M.; Xu, W.; Sahgal, A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer 2012, 118, 2486–2493. [Google Scholar] [CrossRef]
- Soffietti, R.; Kocher, M.; Abacioglu, U.M.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. A European Organisation for Research and Treatment of Cancer Phase III Trial of Adjuvant Whole-Brain Radiotherapy Versus Observation in Patients with One to Three Brain Metastases from Solid Tumors after Surgical Resection or Radiosurgery: Quality-of-Life. J. Clin. Oncol. 2013, 31, 65–72. [Google Scholar] [CrossRef]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Yamamoto, M.; Serizawa, T.; Higuchi, Y.; Sato, Y.; Kawagishi, J.; Yamanaka, K.; Shuto, T.; Akabane, A.; Jokura, H.; Yomo, S.; et al. A Multi-institutional Prospective Observational Study of Stereotactic Radiosurgery for Patients with Multiple Brain Metastases (JLGK0901 Study Update): Irradiation-related Complications and Long-term Maintenance of Mini-Mental State Examination Scores. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, K.R.; Burri, S.H.; Asher, A.L.; Crocker, I.R.; Fraser, R.W.; Zhang, C.; Chen, Z.; Kandula, S.; Zhong, J.; Press, R.H.; et al. Comparing Preoperative With Postoperative Stereotactic Radiosurgery for Resectable Brain Metastases. Neurosurgery 2016, 79, 279–285. [Google Scholar] [CrossRef]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal Avoidance during Whole-Brain Radiotherapy Plus Memantine for Patients with Brain Metastases: Phase III Trial NRG Oncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef]
- Sun, B.; Huang, Z.; Wu, S.; Shen, G.; Cha, L.; Meng, X.; Ding, L.; Wang, J.; Song, S. Incidence and relapse risk of intracranial metastases within the perihippocampal region in 314 patients with breast cancer. Radiother. Oncol. 2016, 118, 181–186. [Google Scholar] [CrossRef]
- Monje, M.L.; Palmer, T. Radiation injury and neurogenesis. Curr. Opin. Neurol. 2003, 16, 129–134. [Google Scholar] [CrossRef]
- Brown, P.D.; Pugh, S.; Laack, N.N.; Wefel, J.S.; Khuntia, D.; Meyers, C.; Choucair, A.; Fox, S.; Suh, J.H.; Roberge, D.; et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013, 15, 1429–1437. [Google Scholar] [CrossRef]
- Lin, N.U.; Diéras, V.; Paul, D.; Lossignol, D.; Christodoulou, C.; Stemmler, H.-J.; Roché, H.; Liu, M.C.; Greil, R.; Ciruelos, E.; et al. Multicenter Phase II Study of Lapatinib in Patients with Brain Metastases from HER2-Positive Breast Cancer. Clin. Cancer Res. 2009, 15, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Bachelot, T.; Romieu, G.; Campone, M.; Dieras, V.; Cropet, C.; Dalenc, F.; Jimenez, M.; Le Rhun, E.; Pierga, J.Y.; Goncalves, A.; et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013, 14, 64–71. [Google Scholar] [CrossRef]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, N.U.; Freedman, R.A.; Ramakrishna, N.; Younger, J.; Storniolo, A.M.; Bellon, J.R.; Come, S.E.; Gelman, R.S.; Harris, G.J.; Henderson, M.A.; et al. A phase I study of lapatinib with whole brain radiotherapy in patients with Human Epidermal Growth Factor Receptor 2 (HER2)-positive breast cancer brain metastases. Breast Cancer Res. Treat. 2013, 142, 405–414. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Miles, D.; Im, Y.H.; Quah, C.; Lee, L.F.; Cortes, J. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: Results from the randomized phase III study CLEOPATRA. Ann. Oncol. 2014, 25, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Lin, N.U.; Blackwell, K.; Guardino, E.; Huober, J.; Lu, M.; Miles, D.; Samant, M.; Welslau, M.; Diéras, V. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: A retrospective, exploratory analysis in EMILIA. Ann. Oncol. 2015, 26, 113–119. [Google Scholar] [CrossRef]
- Montemurro, F.; Delaloge, S.; Barrios, C.H.; Wuerstlein, R.; Anton, A.; Brain, E.; Hatschek, T.; Kelly, C.M.; Peña-Murillo, C.; Yilmaz, M.; et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: Exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial☆. Ann. Oncol. 2020, 31, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Awada, A.; Colomer, R.; Inoue, K.; Bondarenko, I.; Badwe, R.A.; Demetriou, G.; Lee, S.-C.; Mehta, A.O.; Kim, S.-B.; Bachelot, T.; et al. Neratinib Plus Paclitaxel vs Trastuzumab Plus Paclitaxel in Previously Untreated Metastatic ERBB2-Positive Breast Cancer. JAMA Oncol. 2016, 2, 1557. [Google Scholar] [CrossRef]
- Freedman, R.A.; Gelman, R.S.; Anders, C.K.; Melisko, M.E.; Parsons, H.A.; Cropp, A.M.; Silvestri, K.; Cotter, C.M.; Componeschi, K.P.; Marte, J.M.; et al. TBCRC 022: A Phase II Trial of Neratinib and Capecitabine for Patients with Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer and Brain Metastases. J. Clin. Oncol. 2019, 37, 1081–1089. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.-H.; Dai, M.-S.; Chen, S.-W.; Hurvitz, S.A.; Kim, S.-B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef]
- Cortés, J.; Dieras, V.; Ro, J.; Barriere, J.; Bachelot, T.; Hurvitz, S.; Le Rhun, E.; Espie, M.; Kim, S.B.; Schneeweiss, A.; et al. Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): A randomised, open-label, multicentre, phase 2 trial. Lancet Oncol. 2015, 16, 1700–1710. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Jerusalem, G.; Park, Y.H.; Yamashita, T.; Hurvitz, S.A.; Chen, S.; Cathcart, J.; Lee, C.; Perrin, C. 138O CNS metastases in HER2-positive metastatic breast cancer treated with trastuzumab deruxtecan: DESTINY-Breast01 subgroup analyses. Ann. Oncol. 2020, 31, S63–S64. [Google Scholar] [CrossRef]
- Dent, S.; Cortés, J.; Im, Y.H.; Diéras, V.; Harbeck, N.; Krop, I.E.; Wilson, T.R.; Cui, N.; Schimmoller, F.; Hsu, J.Y.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, A.; Johnston, S.; Lee, K.S.; Ciruelos, E.; Lønning, P.E.; Janni, W.; O’Regan, R.; Mouret-Reynier, M.-A.; Kalev, D.; Egle, D.; et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 87–100. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Leone, J.P.; Emblem, K.E.; Weitz, M.; Gelman, R.S.; Schneider, B.P.; Freedman, R.A.; Younger, J.; Pinho, M.C.; Sorensen, A.G.; Gerstner, E.R.; et al. Phase II trial of carboplatin and bevacizumab in patients with breast cancer brain metastases. Breast Cancer Res. 2020, 22, 131. [Google Scholar] [CrossRef]
- Wu, P.-F.; Lin, C.-H.; Kuo, C.-H.; Chen, W.-W.; Yeh, D.-C.; Liao, H.-W.; Huang, S.-M.; Cheng, A.-L.; Lu, Y.-S. A pilot study of bevacizumab combined with etoposide and cisplatin in breast cancer patients with leptomeningeal carcinomatosis. BMC Cancer 2015, 15, s12885–s13015. [Google Scholar] [CrossRef] [Green Version]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Diéras, V.; Han, H.S.; Kaufman, B.; Wildiers, H.; Friedlander, M.; Ayoub, J.-P.; Puhalla, S.L.; Bondarenko, I.; Campone, M.; Jakobsen, E.H.; et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1269–1282. [Google Scholar] [CrossRef]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-Trastuzumab PET Imaging in Patients with HER2-Positive Breast Cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.H.; Park, M.J.; Ji, S.H.; Yi, S.Y.; Lim, D.H.; Nam, D.H.; Lee, J.I.; Park, W.; Choi, D.H.; Huh, S.J.; et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br. J. Cancer 2009, 100, 894–900. [Google Scholar] [CrossRef]
- Pestalozzi, B.C.; Holmes, E.; de Azambuja, E.; Metzger-Filho, O.; Hogge, L.; Scullion, M.; Lang, I.; Wardley, A.; Lichinitser, M.; Sanchez, R.I.; et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: A retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013, 14, 244–248. [Google Scholar] [CrossRef]
- Figura, N.B.; Rizk, V.T.; Armaghani, A.J.; Arrington, J.A.; Etame, A.B.; Han, H.S.; Czerniecki, B.J.; Forsyth, P.A.; Ahmed, K.A. Breast leptomeningeal disease: A review of current practices and updates on management. Breast Cancer Res. Treat. 2019, 177, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, G.; Darrouzain, F.; De Bazelaire, C.; Ternant, D.; Barranger, E.; Winterman, S.; Madelaine-Chambin, I.; Thiebaut, J.-B.; Polivka, M.; Paintaud, G.; et al. Intrathecal Trastuzumab Halts Progression of CNS Metastases in Breast Cancer. J. Clin. Oncol. 2016, 34, e151–e155. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Casey, M.; Press, M.; Lindquist, D.; Pienkowski, T.; Romieu, C.G.; Chan, S.; Jagiello-Gruszfeld, A.; Kaufman, B.; Crown, J.; et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res. Treat. 2008, 112, 533–543. [Google Scholar] [CrossRef]
- Pivot, X.; Manikhas, A.; Żurawski, B.; Chmielowska, E.; Karaszewska, B.; Allerton, R.; Chan, S.; Fabi, A.; Bidoli, P.; Gori, S.; et al. CEREBEL (EGF111438): A Phase III, Randomized, Open-Label Study of Lapatinib Plus Capecitabine Versus Trastuzumab Plus Capecitabine in Patients With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer. J. Clin. Oncol. 2015, 33, 1564–1573. [Google Scholar] [CrossRef]
- Kaplan, M.A.; Isikdogan, A.; Koca, D.; Kucukoner, M.; Gumusay, O.; Yildiz, R.; Dayan, A.; Demir, L.; Geredeli, C.; Kocer, M.; et al. Clinical outcomes in patients who received lapatinib plus capecitabine combination therapy for HER2-positive breast cancer with brain metastasis and a comparison of survival with those who received trastuzumab-based therapy: A study by the Anatolian Socie. Breast Cancer 2014, 21, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Niikura, N.; Masuda, N.; Takashima, S.; Nakamura, R.; Watanabe, K.-I.; Kanbayashi, C.; Ishida, M.; Hozumi, Y.; Tsuneizumi, M.; et al. Prognostic factors of HER2-positive breast cancer patients who develop brain metastasis: A multicenter retrospective analysis. Breast Cancer Res. Treat. 2015, 149, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Gelmon, K.A.; Boyle, F.M.; Kaufman, B.; Huntsman, D.G.; Manikhas, A.; Di Leo, A.; Martin, M.; Schwartzberg, L.S.; Lemieux, J.; Aparicio, S.; et al. Lapatinib or Trastuzumab Plus Taxane Therapy for Human Epidermal Growth Factor Receptor 2–Positive Advanced Breast Cancer: Final Results of NCIC CTG MA.31. J. Clin. Oncol. 2015, 33, 1574–1583. [Google Scholar] [CrossRef]
- Sambade, M.J.; Kimple, R.J.; Camp, J.T.; Peters, E.; Livasy, C.A.; Sartor, C.I.; Shields, J.M. Lapatinib in Combination With Radiation Diminishes Tumor Regrowth in HER2+ and Basal-Like/EGFR+ Breast Tumor Xenografts. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 575–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swain, S.M.; Kim, S.-B.; Cortés, J.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Knott, A.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013, 14, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Lin, N.U.; Stein, A.; Nicholas, A.; Fung, A.M.; Kumthekar, P.; Ibrahim, N.K.; Pegram, M.D. Planned interim analysis of PATRICIA: An open-label, single-arm, phase II study of pertuzumab (P) with high-dose trastuzumab (H) for the treatment of central nervous system (CNS) progression post radiotherapy (RT) in patients (pts) with HER2-positive metastatic breast cancer (MBC). J. Clin. Oncol. 2017, 35, 2074. [Google Scholar] [CrossRef]
- Bartsch, R.; Berghoff, A.S.; Vogl, U.; Rudas, M.; Bergen, E.; Dubsky, P.; Dieckmann, K.; Pinker, K.; Bago-Horvath, Z.; Galid, A.; et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin. Exp. Metastasis 2015, 32, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Jacot, W.; Pons, E.; Frenel, J.-S.; Guiu, S.; Levy, C.; Heudel, P.E.; Bachelot, T.; D’Hondt, V.; Darlix, A.; Firmin, N.; et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res. Treat. 2016, 157, 307–318. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Rabindran, S.K.; Discafani, C.M.; Rosfjord, E.C.; Baxter, M.; Floyd, M.B.; Golas, J.; Hallett, W.A.; Johnson, B.D.; Nilakantan, R.; Overbeek, E.; et al. Antitumor Activity of HKI-272, an Orally Active, Irreversible Inhibitor of the HER-2 Tyrosine Kinase. Cancer Res. 2004, 64, 3958–3965. [Google Scholar] [CrossRef] [Green Version]
- Burstein, H.J.; Sun, Y.; Dirix, L.Y.; Jiang, Z.; Paridaens, R.; Tan, A.R.; Awada, A.; Ranade, A.; Jiao, S.; Schwartz, G.; et al. Neratinib, an Irreversible ErbB Receptor Tyrosine Kinase Inhibitor, in Patients With Advanced ErbB2-Positive Breast Cancer. J. Clin. Oncol. 2010, 28, 1301–1307. [Google Scholar] [CrossRef]
- Chow, L.W.C.; Xu, B.; Gupta, S.; Freyman, A.; Zhao, Y.; Abbas, R.; Vo Van, M.L.; Bondarenko, I. Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer. Br. J. Cancer 2013, 108, 1985–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.; Holmes, F.A.; Ejlertsen, B.; Delaloge, S.; Moy, B.; Iwata, H.; Von Minckwitz, G.; Chia, S.K.L.; Mansi, J.; Barrios, C.H.; et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1688–1700. [Google Scholar] [CrossRef]
- Chan, A.; Moy, B.; Mansi, J.; Ejlertsen, B.; Holmes, F.A.; Chia, S.; Iwata, H.; Gnant, M.; Loibl, S.; Barrios, C.H.; et al. Final Efficacy Results of Neratinib in HER2-positive Hormone Receptor-positive Early-stage Breast Cancer From the Phase III ExteNET Trial. Clin. Breast Cancer 2020, 10, 1016. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Rusz, O.; Kószó, R.; Dobi, Á.; Csenki, M.; Valicsek, E.; Nikolényi, A.; Uhercsák, G.; Cserháti, A.; Kahán, Z. Clinical benefit of fulvestrant monotherapy in the multimodal treatment of hormone receptor and HER2 positive advanced breast cancer: A case series. Onco Targets 2018, 11, 5459–5463. [Google Scholar] [CrossRef] [Green Version]
- Network, T.C.G.A. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Rhun, E.; Bertrand, N.; Dumont, A.; Tresch, E.; Le Deley, M.C.; Mailliez, A.; Preusser, M.; Weller, M.; Revillion, F.; Bonneterre, J. Identification of single nucleotide polymorphisms of the PI3K-AKT-mTOR pathway as a risk factor of central nervous system metastasis in metastatic breast cancer. Eur. J. Cancer 2017, 87, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, I.C.; Hsiao, L.-P.; Huang, I.W.; Yu, H.-C.; Yeh, L.-C.; Lin, C.-H.; Wei-Wu Chen, T.; Cheng, A.-L.; Lu, Y.-S. Phosphatidylinositol-3 Kinase Inhibitors, Buparlisib and Alpelisib, Sensitize Estrogen Receptor-positive Breast Cancer Cells to Tamoxifen. Sci. Rep. 2017, 7, 9842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batalini, F.; Moulder, S.L.; Winer, E.P.; Rugo, H.S.; Lin, N.U.; Wulf, G.M. Response of Brain Metastases From PIK3CA-Mutant Breast Cancer to Alpelisib. Jco Precis. Oncol. 2020, 10, 572–578. [Google Scholar] [CrossRef]
- Jin, X.; Demere, Z.; Nair, K.; Ali, A.; Ferraro, G.B.; Natoli, T.; Deik, A.; Petronio, L.; Tang, A.A.; Zhu, C.; et al. A metastasis map of human cancer cell lines. Nature 2020, 588, 331–336. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Sahebjam, S.; Le Rhun, E.; Bachelot, T.; Kabos, P.; Awada, A.; Yardley, D.; Chan, A.; Conte, P.; Diéras, V.; et al. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor–Positive Breast Cancer. Clin. Cancer Res. 2020, 26, 5310–5319. [Google Scholar] [CrossRef]
- Santa-Maria, C.A.; Kumthekar, P.; Rademaker, A.; Gross, L.; Jain, S.; Flaum, L.E.; Gradishar, W.J.; Cristofanilli, M. A pilot study of palbociclib in patients with HER2-positive breast cancer with brain metastasis. J. Clin. Oncol. 2017, 35, TPS1110. [Google Scholar] [CrossRef]
- Duchnowska, R.; Pęksa, R.; Radecka, B.; Mandat, T.; Trojanowski, T.; Jarosz, B.; Czartoryska-Arłukowicz, B.; Olszewski, W.P.; Och, W.; Kalinka-Warzocha, E.; et al. Immune response in breast cancer brain metastases and their microenvironment: The role of the PD-1/PD-L axis. Breast Cancer Res. 2016, 18, s13058–s14016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luen, S.J.; Salgado, R.; Fox, S.; Savas, P.; Eng-Wong, J.; Clark, E.; Kiermaier, A.; Swain, S.M.; Baselga, J.; Michiels, S.; et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: A retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017, 18, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.; Lee, S.J.; Kim, Y.K.; Park, W.Y.; Park, D.Y.; Kim, J.Y.; Lee, C.H.; Gong, G.; Huh, G.Y.; Choi, K.U. Programmed death-ligand 1 (PD-L1) expression in tumour cell and tumour infiltrating lymphocytes of HER2-positive breast cancer and its prognostic value. Sci. Rep. 2017, 7, s41598–s42017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narloch, J.; Luedke, C.; Broadwater, G.; Priedigkeit, N.; Hall, A.; Hyslop, T.; Sammons, S.L.; Huggins-Puhalla, S.L.; Leone, J.P.; Ramirez, J.; et al. Number of tumor-infiltrating lymphocytes in breast cancer brain metastases compared to matched breast primaries. J. Clin. Oncol. 2017, 35, 2049. [Google Scholar] [CrossRef]
- Li, Y.; Vennapusa, B.; Chang, C.W.; Tran, D.; Nakamura, R.; Sumiyoshi, T.; Hegde, P.; Molinero, L. Prevalence Study of PD-L1 SP142 Assay in Metastatic Triple-negative Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2020, 10, 1097. [Google Scholar] [CrossRef]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.-T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [Green Version]
- Mehta, M.P.; Wang, D.; Wang, F.; Kleinberg, L.; Brade, A.; Robins, H.I.; Turaka, A.; Leahy, T.; Medina, D.; Xiong, H.; et al. Veliparib in combination with whole brain radiation therapy in patients with brain metastases: Results of a phase 1 study. J. Neurooncol. 2015, 122, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.J.; Giannoudis, A.; Palmieri, C. The genomic landscape of breast cancer brain metastases: A systematic review. Lancet Oncol. 2021, 22, e7–e17. [Google Scholar] [CrossRef]
- Duchnowska, R.; Dziadziuszko, R.; Trojanowski, T.; Mandat, T.; Och, W.; Czartoryska-Arłukowicz, B.; Radecka, B.; Olszewski, W.; Szubstarski, F.; Kozłowski, W.; et al. Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res. 2012, 14, R119. [Google Scholar] [CrossRef] [Green Version]
- Schrijver, W.A.M.E.; Suijkerbuijk, K.P.M.; Van Gils, C.H.; Van Der Wall, E.; Moelans, C.B.; Van Diest, P.J. Receptor Conversion in Distant Breast Cancer Metastases: A Systematic Review and Meta-analysis. Jnci. J. Natl. Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulsbergen, A.F.C.; Claes, A.; Kavouridis, V.K.; Ansaripour, A.; Nogarede, C.; Hughes, M.E.; Smith, T.R.; Brastianos, P.K.; Verhoeff, J.J.C.; Lin, N.U.; et al. Subtype switching in breast cancer brain metastases: A multicenter analysis. Neuro Oncol. 2020, 22, 1173–1181. [Google Scholar] [CrossRef]
- Palmieri, D.; Bronder, J.L.; Herring, J.M.; Yoneda, T.; Weil, R.J.; Stark, A.M.; Kurek, R.; Vega-Valle, E.; Feigenbaum, L.; Halverson, D.; et al. Her-2 Overexpression Increases the Metastatic Outgrowth of Breast Cancer Cells in the Brain. Cancer Res. 2007, 67, 4190–4198. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.; Simpson, P.T.; Smart, C.E.; Cocciardi, S.; Waddell, N.; Lane, A.; Morrison, B.J.; Vargas, A.C.; Healey, S.; Beesley, J.; et al. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res. 2010, 12, R46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, A.H.; McGrane, J.; Mathew, J.; Palmer, J.; Hilton, D.A.; Purvis, G.; Jenkins, R. Changing molecular profile of brain metastases compared with matched breast primary cancers and impact on clinical outcomes. Br. J. Cancer 2016, 114, 793–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priedigkeit, N.; Hartmaier, R.J.; Chen, Y.; Vareslija, D.; Basudan, A.; Watters, R.J.; Thomas, R.; Leone, J.P.; Lucas, P.C.; Bhargava, R.; et al. Intrinsic Subtype Switching and Acquired ERBB2/HER2 Amplifications and Mutations in Breast Cancer Brain Metastases. JAMA Oncol. 2017, 3, 666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.P.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.M.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Nigro, C.; Vivenza, D.; Monteverde, M.; Lattanzio, L.; Gojis, O.; Garrone, O.; Comino, A.; Merlano, M.; Quinlan, P.R.; Syed, N.; et al. High frequency of complex TP53 mutations in CNS metastases from breast cancer. Br. J. Cancer 2012, 106, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Park, K.; Lim, S.H.; Kim, H.S.; Yoo, K.H.; Jung, K.S.; Song, H.-N.; Hong, M.; Do, I.-G.; Ahn, T.; et al. Mutational profiling of brain metastasis from breast cancer: Matched pair analysis of targeted sequencing between brain metastasis and primary breast cancer. Oncotarget 2015, 6, 43731–43742. [Google Scholar] [CrossRef] [Green Version]
- Tyran, M.; Carbuccia, N.; Garnier, S.; Guille, A.; Adelaïde, J.; Finetti, P.; Touzlian, J.; Viens, P.; Tallet, A.; Goncalves, A.; et al. A Comparison of DNA Mutation and Copy Number Profiles of Primary Breast Cancers and Paired Brain Metastases for Identifying Clinically Relevant Genetic Alterations in Brain Metastases. Cancers 2019, 11, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, R.; Nakano, T.; Hosonaga, M.; Sampetrean, O.; Harigai, R.; Sasaki, T.; Koya, I.; Okano, H.; Kudoh, J.; Saya, H.; et al. RNA Sequencing Analysis Reveals Interactions between Breast Cancer or Melanoma Cells and the Tissue Microenvironment during Brain Metastasis. Biomed. Res. Int. 2017, 2017, 8032910. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Dang, Y.; Shao, X.; Chen, X.; Wu, F.; Li, Y. Ubiquitination and Long Non-coding RNAs Regulate Actin Cytoskeleton Regulators in Cancer Progression. Int. J. Mol. Sci. 2019, 20, 2997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Liang, K.; Hu, Q.; Li, P.; Song, J.; Yang, Y.; Yao, J.; Mangala, L.S.; Li, C.; Yang, W.; et al. JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J. Clin. Investig. 2017, 127, 4498–4515. [Google Scholar] [CrossRef] [Green Version]
- Xing, F.; Liu, Y.; Wu, S.-Y.; Wu, K.; Sharma, S.; Mo, Y.-Y.; Feng, J.; Sanders, S.; Jin, G.; Singh, R.; et al. Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal miRNA to Promote Brain Metastasis. Cancer Res. 2018, 78, 4316–4330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanchan, R.K.; Siddiqui, J.A.; Mahapatra, S.; Batra, S.K.; Nasser, M.W. microRNAs Orchestrate Pathophysiology of Breast Cancer Brain Metastasis: Advances in Therapy. Mol. Cancer 2020, 19, s12943–s13020. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuda, H.; Xing, F.; Pandey, P.R.; Sharma, S.; Watabe, M.; Pai, S.K.; Mo, Y.-Y.; Iiizumi-Gairani, M.; Hirota, S.; Liu, Y.; et al. miR-7 Suppresses Brain Metastasis of Breast Cancer Stem-Like Cells By Modulating KLF4. Cancer Res. 2013, 73, 1434–1444. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Sullivan, P.S.; Goodman, J.C.; Gunaratne, P.H.; Marchetti, D. MicroRNA-1258 Suppresses Breast Cancer Brain Metastasis by Targeting Heparanase. Cancer Res. 2011, 71, 645–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, F.; Sharma, S.; Liu, Y.; Mo, Y.Y.; Wu, K.; Zhang, Y.Y.; Pochampally, R.; Martinez, L.A.; Lo, H.W.; Watabe, K. miR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-α. Oncogene 2015, 34, 4890–4900. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Ginnebaugh, K.R.; Sethi, S.; Chen, W.; Ali, R.; Mittal, S.; Sarkar, F.H. miR-20b is up-regulated in brain metastases from primary breast cancers. Oncotarget 2015, 6, 12188–12195. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.-C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015, 527, 100–104. [Google Scholar] [CrossRef]
- Reijerkerk, A.; Lopez-Ramirez, M.A.; Van Het Hof, B.; Drexhage, J.A.R.; Kamphuis, W.W.; Kooij, G.; Vos, J.B.; Van Der Pouw Kraan, T.C.T.M.; Van Zonneveld, A.J.; Horrevoets, A.J.; et al. MicroRNAs Regulate Human Brain Endothelial Cell-Barrier Function in Inflammation: Implications for Multiple Sclerosis. J. Neurosci. 2013, 33, 6857–6863. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood–brain barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Zhang, Y.; Hua, J.; Yang, X.; Zhang, X.; Duan, M.; Zhu, X.; Huang, W.; Chao, J.; Zhou, R.; et al. Silencing microRNA-143 protects the integrity of the blood-brain barrier: Implications for methamphetamine abuse. Sci. Rep. 2016, 6, 35642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debeb, B.G.; Lacerda, L.; Anfossi, S.; Diagaradjane, P.; Chu, K.; Bambhroliya, A.; Huo, L.; Wei, C.; Larson, R.A.; Wolfe, A.R.; et al. miR-141-Mediated Regulation of Brain Metastasis From Breast Cancer. J. Natl. Cancer Inst. 2016, 108, djw026. [Google Scholar] [CrossRef] [Green Version]
- Teplyuk, N.M.; Mollenhauer, B.; Gabriely, G.; Giese, A.; Kim, E.; Smolsky, M.; Kim, R.Y.; Saria, M.G.; Pastorino, S.; Kesari, S.; et al. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro Oncol. 2012, 14, 689–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.J.; Alexander, B.; Schnitt, S.J.; Comander, A.; Gallagher, B.; Garber, J.E.; Tung, N. Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and noncarriers. Cancer 2011, 117, 3093–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albiges, L.; André, F.; Balleyguier, C.; Gomez-Abuin, G.; Chompret, A.; Delaloge, S. Spectrum of breast cancer metastasis in BRCA1 mutation carriers: Highly increased incidence of brain metastases. Ann. Oncol. 2005, 16, 1846–1847. [Google Scholar] [CrossRef]
- Song, Y.; Barry, W.T.; Seah, D.S.; Tung, N.M.; Garber, J.E.; Lin, N.U. Patterns of recurrence and metastasis in BRCA1/BRCA2 -associated breast cancers. Cancer 2020, 126, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavitsanos, P.J.; Wazer, D.E.; Hepel, J.T.; Wang, Y.; Singh, K.; Leonard, K.L. BRCA1 Mutations Associated With Increased Risk of Brain Metastases in Breast Cancer: A 1: 2 Matched-pair Analysis. Am. J. Clin. Oncol. 2018, 41, 1252–1256. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Breast Cancer (Version 1.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 2 March 2021).
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smerage, J.B.; Barlow, W.E.; Hortobagyi, G.N.; Winer, E.P.; Leyland-Jones, B.; Srkalovic, G.; Tejwani, S.; Schott, A.F.; O’Rourke, M.A.; Lew, D.L.; et al. Circulating Tumor Cells and Response to Chemotherapy in Metastatic Breast Cancer: SWOG S0500. J. Clin. Oncol. 2014, 32, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- De Mattos-Arruda, L.; Mayor, R.; Ng, C.K.Y.; Weigelt, B.; Martínez-Ricarte, F.; Torrejon, D.; Oliveira, M.; Arias, A.; Raventos, C.; Tang, J.; et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 2015, 6, 8839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siravegna, G.; Geuna, E.; Mussolin, B.; Crisafulli, G.; Bartolini, A.; Galizia, D.; Casorzo, L.; Sarotto, I.; Scaltriti, M.; Sapino, A.; et al. Genotyping tumour DNA in cerebrospinal fluid and plasma of a HER2-positive breast cancer patient with brain metastases. Esmo Open 2017, 2, e000253. [Google Scholar] [CrossRef] [Green Version]
- Boire, A.; Brandsma, D.; Brastianos, P.K.; Le Rhun, E.; Ahluwalia, M.; Junck, L.; Glantz, M.; Groves, M.D.; Lee, E.Q.; Lin, N.; et al. Liquid biopsy in central nervous system metastases: A RANO review and proposals for clinical applications. Neuro Oncol. 2019, 21, 571–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Treatment Type | Patients’ Population | Author | Trial Name (NCT Number) | Phase | Primary Endpoint |
|---|---|---|---|---|---|
| Surgery plus WBRT vs. WBRT | Single BM | Patchell et al. [39] | III | OS | |
| Surgery plus WBRT vs. Surgery alone | Single BM | Patchell et al. [40] | III | Recurrence of tumor in the brain | |
| Postoperative SRS vs. WBRT | Single BM (a resected BM and a resection cavity less than 5.0cm) | Brown et al. [41] | NCCTG N107C/CEC·3 (NCT01372774) | III | Cognitive-deterioration-free survival and OS |
| Salvage SRS vs. postoperative WBRT | 1 to 4 resected BMs with only one lesion > 3 cm | Kayama et al. [42] | JCOG0504 | III | OS |
| WBRT alone vs. WBRT followed by SRS | 1 to 3 BMs | Andrews et al. [43] | RTOG9508 (NCT00002708) | III | OS |
| SRS or Surgery with/without WBRT | 1 to 3 BMs | Kocher et al. [44] | EORTC 22952-26001 (NCT00002899) | III | Time to PS deterioration more than 2 |
| SRS alone vs. SRS plus WBRT | 1 to 3 BMs | Brown et al. [45] | (NCT00377156) | III | Cognitive deterioration at 3 months |
| SRS plus WBRT vs. SRS alone | 1 to 4 BMs, each under than 3 cm | Aoyama et al. [46] | (C000000412) *,1 | III | OS |
| SRS for 2–4 BMs vs. 5–10 BMs | Patients with BMs who received SRS | Yamamoto et al. [47] | JLGK0901 (UMIN000001812) *,1 | III | OS |
| SRS vs WBRT | 4–15 untreated non-melanoma BMs | Li et al. [48] | (NCT01592968) | III | Local control rate and proportion of patients with neurocognitive decline at 4 months |
| SRS vs HA-WBRT | 5–20 BMs | (Recruiting) | (NCT03075072) | III | Quality of life |
| HA-WBRT | BM outside a 5 mm margin around either hippocampus | Gondi et al. [49] | RTOG 0933 (NCT01227954) | II | Cognitive function |
| Systemic therapy for HER2-positive BCBM | |||||
| Treatment | Patients’ Population | Author | Trial Name (NCT Number) | Phase | Primary Endpoint |
| Lapatinib | Progressive HER2-positive BCBM after prior trastuzumab, and cranial radiotherapy | Lin et al. [65] | EGF 105084 (NCT00263588) | II | ORR in CNS |
| Lapatinib plus capecitabine | HER2-positive BCBM not previously treated with WBRT, capecitabine, or lapatinib | Bachelot et al. [66] | LANDSCAPE (NCT00967031) | II | ORR in CNS |
| Lapatinib plus capecitabine vs. capecitabine alone | HER2-positive, locally advanced or MBC that had progressed after treatment with regimens that included an anthracycline, a taxane, and trastuzumab | Geyer et al. [67] | EGF100151 (NCT00078572) | III | Time to progression |
| Lapatinib plus WBRT | HER2-positive BCBM | Lin et al. [68] | (not available) | I | Maximum tolerated dose of concurrent lapatinib with WBRT |
| Pertuzumab plus trastuzumab and docetaxel vs trastuzumab plus docetaxel | HER2-positive locally recurrent, unresectable, or MBC without prior chemotherapy or biologic therapy for their advanced disease | Swain et al. [69] | CLEOPATRA (NCT00567190) | III | PFS |
| T-DM1 vs. lapatinb plus capecitabine | HER2-positive advanced breast cancer previously treated with trastuzumab and a taxane | Krop et al. [70]. | EMILIA (NCT00829166) | III | Percentage of participants with progressive disease or death, PFS, OS, et al. |
| T-DM1 | HER2-positive locally advanced or MBC with prior HER2-targeted therapy and chemotherapy | Montemurro et al. [71] | KAMILLA (NCT01702571) | III | Best overall response rate, clinical benefit rate |
| Neratinib plus paclitaxel vs. trastuzumab plus paclitaxel | Previously untreated recurrent and/or metastatic HER2-positive BC | Awada et al. [72] | NEfERT-T (NCT00915018) | III | PFS |
| Neratinib plus capecitabine | Measurable, progressive, HER2-positive BCBM | Freedman et al. [73] | TBCRC 022 (NCT01494662) | II | ORR |
| Neratinib plus capecitabine vs. lapatinib plus capecitabine | HER2-positive MBC with 2 or more previous HER2-directed MBC regimens. | Saura et al. [74] | NALA trial (NCT01808573) | III | PFS, OS |
| Afatinib alone vs. afatinib plus vinorelbine vs. investigator’s choice | HER2-positive BCBM with recurrence or progression during or after treatment with trastuzumab, lapatinib, or both | Cortés et al. [75] | LUX-Breast 3 (NCT01441596) | II | Patient benefit at 12 weeks |
| Tucatinib | HER2-positive MBC previously treated with trastuzumab, pertuzumab, and trastuzumab emtansine | Murthy et al. [76] | HER2CLIMB (NCT02614794) | II | PFS |
| Tucatinib plus T-DM1 vs. T-DM1 | HER2-positive MBC previously treated with a taxane and/or trastuzumab | (Ongoing) | HER2CLIMB-02 (NCT03975647) | III | PFS |
| Trastuzumab Deruxtecan | HER2-positive metastatic breast cancer who had received previous treatment with trastuzumab emtansine | Jerusalem et al. [77] | DESTINY-Breast01 (NCT03248492) | II | ORR |
| Systemic therapy for luminal-type BCBM | |||||
| Treatment | Patients’ Population | Author | Trial Name (NCT Number) | Phase | Primary Endpoint |
| Taselisib plus fulvestrant vs. fulvestrant alone | ER-positive and HER2-negative locally advanced BC or MBC with recurrence or progression after aromatase inhibitor therapy | Dent et al. [78] | SANDPIPER (NCT02340221) | III | PFS |
| Alpelisib plus fulvestrant vs. fulvestrant alone | HR-positive, HER2-negative, advanced BC with progression after aromatase inhibitor therapy | André et al. [79] | SOLAR-1 (NCT02437318) | III | PFS |
| Buparlisib plus fulvestrant vs. fulvestrant alone | HR-positive, HER2-negative, locally advanced or metastatic breast cancer, who had relapsed on or after endocrine therapy and mTOR inhibitors | Di Leo et al. [80] | BELLE-3 (NCT01633060) | III | PFS |
| Palbociclib | Measurable progressive luminal-type BCBM | (Ongoing) | (NCT02896335) | II | Clinical benefit rate at 8 weeks |
| Abemaciclib | BM from luminal-type BC, NSCLC, or melanoma | (Ongoing) | (NCT02308020) | II | ORR in CNS |
| Palbociclib plus trastuzumab plus lapatinib plus fulvestrant | ER-positive/HER2-positive BCBM | (Ongoing) | (NCT04334330) | ORR | |
| Abemaciblib plus SRS vs. palbociclib plus SRS vs. ribociclib plus SRS | ER-positive/HER-2 negative BCBM | (Ongoing) | (NCT04585724) | I | Incidence of grade 3+ radiation therapy oncology central nervous system toxicity |
| Systemic therapy for TN-type BCBM | |||||
| Treatment | Patients’ Population | Author | Trial Name (NCT Number) | Phase | Primary Endpoint |
| Pembrolizumab | Advance TNBC, advanced head and neck cancer, advanced urothelial cancer, or advanced gastric cancer | Nanda et al. [81] | KEYNOTE-012 (NCT01848834) | I | Adverse events and overall response rate |
| Atezolizumab plus nab-paclitaxel vs. nab-paclitaxel | unresectable locally advanced or metastatic TNBC | Schmid et al. [82] | IMpassion130 (NCT02425891) | III | PFS and OS |
| Carboplatin and bevacizumab | New or progressive BCBM | Leone et al. [83] | (NCT01004172) | II | ORR in CNS |
| Bevacizumab, etoposide, cisplatin | Breast cancer brain and/or leptomeningeal metastasis | Wu et al. [84] | (NCT01281696) | II | ORR in CNS |
| Talazoparib vs. single agent chemotherapy investigator’s choice | Advanced and/or MBC patients with BRCA mutation, which received no more than 3 prior chemotherapy-inclusive regimens for locally advanced and/or metastatic disease | Litton et al. [85] | EMBRACA (NCT01945775) | III | PFS |
| Olaparib vs. single agent chemotherapy investigator’s choice | MBC who had received no more than two previous chemotherapy regimens for metastatic disease | Robson et al. [86] | OlympiAD (NCT02000622) | III | PFS |
| Veliparib plus carboplatin plus paclitaxel vs. carboplatin plus paclitaxel | Advanced HER2-negative breast cancer with BRCA1 or BRCA2 mutation | Diéras et al. [87] | BROCADE3 (NCT02163694) | III | PFS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watase, C.; Shiino, S.; Shimoi, T.; Noguchi, E.; Kaneda, T.; Yamamoto, Y.; Yonemori, K.; Takayama, S.; Suto, A. Breast Cancer Brain Metastasis—Overview of Disease State, Treatment Options and Future Perspectives. Cancers 2021, 13, 1078. https://doi.org/10.3390/cancers13051078
Watase C, Shiino S, Shimoi T, Noguchi E, Kaneda T, Yamamoto Y, Yonemori K, Takayama S, Suto A. Breast Cancer Brain Metastasis—Overview of Disease State, Treatment Options and Future Perspectives. Cancers. 2021; 13(5):1078. https://doi.org/10.3390/cancers13051078
Chicago/Turabian StyleWatase, Chikashi, Sho Shiino, Tatsunori Shimoi, Emi Noguchi, Tomoya Kaneda, Yusuke Yamamoto, Kan Yonemori, Shin Takayama, and Akihiko Suto. 2021. "Breast Cancer Brain Metastasis—Overview of Disease State, Treatment Options and Future Perspectives" Cancers 13, no. 5: 1078. https://doi.org/10.3390/cancers13051078
APA StyleWatase, C., Shiino, S., Shimoi, T., Noguchi, E., Kaneda, T., Yamamoto, Y., Yonemori, K., Takayama, S., & Suto, A. (2021). Breast Cancer Brain Metastasis—Overview of Disease State, Treatment Options and Future Perspectives. Cancers, 13(5), 1078. https://doi.org/10.3390/cancers13051078