Assessment of Mixed Plasmodium falciparum sera5 Infection in Endemic Burkitt Lymphoma: A Case-Control Study in Malawi
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Ethics Review
2.3. Laboratory Methods
2.3.1. Pfsera5 Gene Amplification
2.3.2. Pfsera5 Nucleotide Sequencing
2.3.3. Sequence Alignment and Pf Population Genetics Analyses
2.3.4. Statistical Analyses
3. Results
3.1. Characteristics of Study Subjects
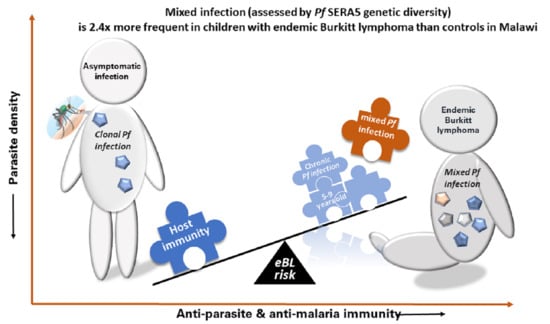
3.2. Association of Mixed Pfsera5 Infection with eBL
3.3. Pfsera5 Haplotype Diversity in eBL Cases and Controls in Malawi
3.4. Patterns of Common Versus Rare Pfsera5 Haplotypes in eBL Cases and Controls
3.5. Genetic Differentiation between Parasite Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burkitt, D. A sarcoma involving the jaws in african children. Br. J. Surg. 1958, 46, 218–223. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.A.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Straif, K. Carcinogenicity of malaria and of some polyomaviruses. Lancet Oncol. 2012, 13, 339–340. [Google Scholar] [CrossRef]
- Stiller, C.A.; Parkin, D.M. International variations in the incidence of childhood lymphomas. Paediatr. Perinat. Epidemiol. 1990, 4, 303–324. [Google Scholar] [CrossRef]
- Hämmerl, L.; Colombet, M.; Rochford, R.; Ogwang, D.M.; Parkin, D.M. The burden of Burkitt lymphoma in Africa. Infect. Agents Cancer 2019, 14, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Linet, M.S.; Brown, L.M.; Mbulaiteye, S.M.; Check, D.; Ostroumova, E.; Landgren, A.; Devesa, S.S. International long-term trends and recent patterns in the incidence of leukemias and lymphomas among children and adolescents ages 0–19 years. Int. J. Cancer 2016, 138, 1862–1874. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, L.M.; Newton, R.; Casabonne, D.; Ziegler, J.; Mbulaiteye, S.; Mbidde, E.; Wabinga, H.; Jaffe, H.; Beral, V. Antibodies against malaria and epstein-barr virus in childhood burkitt lymphoma: A case-control study in uganda. Int. J. Cancer 2008, 122, 1319–1323. [Google Scholar] [CrossRef]
- Aka, P.; Vila, M.C.; Jariwala, A.; Nkrumah, F.; Emmanuel, B.; Yagi, M.; Palacpac, N.M.; Periago, M.V.; Neequaye, J.; Kiruthu, C.; et al. Endemic burkitt lymphoma is associated with strength and diversity of plasmodium falciparum malaria stage-specific antigen antibody response. Blood 2013, 122, 629–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derkach, A.; Otim, I.; Pfeiffer, R.M.; Onabajo, O.O.; Legason, I.D.; Nabalende, H.; Ogwang, M.D.; Kerchan, P.; Talisuna, A.O.; Ayers, L.W.; et al. Associations between igg reactivity to plasmodium falciparum erythrocyte membrane protein 1 (pfemp1) antigens and burkitt lymphoma in ghana and uganda case-control studies. EBioMedicine 2019, 39, 358–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legason, I.D.; Pfeiffer, R.M.; Udquim, K.I.; Bergen, A.W.; Gouveia, M.H.; Kirimunda, S.; Otim, I.; Karlins, E.; Kerchan, P.; Nabalende, H.; et al. Evaluating the causal link between malaria infection and endemic burkitt lymphoma in northern uganda: A mendelian randomization study. EBioMedicine 2017, 25, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.O. Haemoglobin genotypes, abo blood groups, and burkitt’s tumour. J. Med. Genet. 1966, 3, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Robbiani, D.F.; Deroubaix, S.; Feldhahn, N.; Oliveira, T.Y.; Callen, E.; Wang, Q.; Jankovic, M.; Silva, I.T.; Rommel, P.C.; Bosque, D.; et al. Plasmodium infection promotes genomic instability and aid-dependent b cell lymphoma. Cell 2015, 162, 727–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torgbor, C.; Awuah, P.; Deitsch, K.; Kalantari, P.; Duca, K.A.; Thorley-Lawson, D.A. A multifactorial role for p. Falciparum malaria in endemic burkitt’s lymphoma pathogenesis. PLoS Pathog. 2014, 10, e1004170. [Google Scholar] [CrossRef] [Green Version]
- Virus, E.B. Epstein-Barr Virus and Kaposi’s Sarcoma Herpesvirus/Human Herpesvirus 8. In Proceedings of the IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Lyon, France, 17–24 June 1997. [Google Scholar]
- Emmanuel, B.; Kawira, E.; Ogwang, M.D.; Wabinga, H.; Magatti, J.; Nkrumah, F.; Neequaye, J.; Bhatia, K.; Brubaker, G.; Biggar, R.J.; et al. African burkitt lymphoma: Age-specific risk and correlations with malaria biomarkers. Am. J. Trop. Med. Hyg. 2011, 84, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Daniels, R.; Volkman, S.K.; Milner, D.A.; Mahesh, N.; Neafsey, D.E.; Park, D.J.; Rosen, D.; Angelino, E.; Sabeti, P.C.; Wirth, D.F.; et al. A general snp-based molecular barcode for plasmodium falciparum identification and tracking. Malar. J. 2008, 7, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, W.T.; Mutalima, N.; Sun, D.; Emmanuel, B.; Bhatia, K.; Aka, P.; Wu, X.; Borgstein, E.; Liomba, G.N.; Kamiza, S.; et al. Relationship between plasmodium falciparum malaria prevalence, genetic diversity and endemic burkitt lymphoma in malawi. Sci. Rep. 2014, 4, 3741. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; Tettelin, H.; Carucci, D.J.; Cummings, L.M.; Aravind, L.; Koonin, E.V.; Shallom, S.; Mason, T.; Yu, K.; Fujii, C.; et al. Chromosome 2 sequence of the human malaria parasite plasmodium falciparum. Science 1998, 282, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Okech, B.; Mujuzi, G.; Ogwal, A.; Shirai, H.; Horii, T.; Egwang, T.G. High titers of igg antibodies against plasmodium falciparum serine repeat antigen 5 (sera5) are associated with protection against severe malaria in ugandan children. Am. J. Trop. Med. Hyg. 2006, 74, 191–197. [Google Scholar] [CrossRef]
- Palacpac, N.M.; Ntege, E.; Yeka, A.; Balikagala, B.; Suzuki, N.; Shirai, H.; Yagi, M.; Ito, K.; Fukushima, W.; Hirota, Y.; et al. Phase 1b randomized trial and follow-up study in uganda of the blood-stage malaria vaccine candidate bk-se36. PLoS ONE 2013, 8, e64073. [Google Scholar] [CrossRef] [Green Version]
- Palacpac, N.M.; Arisue, N.; Tougan, T.; Ishii, K.J.; Horii, T. Plasmodium falciparum serine repeat antigen 5 (se36) as a malaria vaccine candidate. Vaccine 2011, 29, 5837–5845. [Google Scholar] [CrossRef]
- Arisue, N.; Palacpac, N.M.Q.; Tougan, T.; Horii, T. Characteristic features of the sera multigene family in the malaria parasite. Parasites Vectors 2020, 13, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debrabant, A.; Maes, P.; Delplace, P.; Dubremetz, J.F.; Tartar, A.; Camus, D. Intramolecular mapping of plasmodium falciparum p126 proteolytic fragments by n-terminal amino acid sequencing. Mol. Biochem. Parasitol. 1992, 53, 89–95. [Google Scholar] [CrossRef]
- Li, J.; Matsuoka, H.; Mitamura, T.; Horii, T. Characterization of proteases involved in the processing of plasmodium falciparum serine repeat antigen (sera). Mol. Biochem. Parasitol. 2002, 120, 177–186. [Google Scholar] [CrossRef]
- Yeoh, S.; O’Donnell, R.A.; Koussis, K.; Dluzewski, A.R.; Ansell, K.H.; Osborne, S.A.; Hackett, F.; Withers-Martinez, C.; Mitchell, G.H.; Bannister, L.H.; et al. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell 2007, 131, 1072–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stallmach, R.; Kavishwar, M.; Withers-Martinez, C.; Hackett, F.; Collins, C.R.; Howell, S.A.; Yeoh, S.; Knuepfer, E.; Atid, A.J.; Holder, A.A.; et al. Plasmodium falciparum sera5 plays a non-enzymatic role in the malarial asexual blood-stage lifecycle. Mol. Microbiol. 2015, 96, 368–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagi, M.; Bang, G.; Tougan, T.; Palacpac, N.M.; Arisue, N.; Aoshi, T.; Matsumoto, Y.; Ishii, K.J.; Egwang, T.G.; Druilhe, P.; et al. Protective epitopes of the plasmodium falciparum sera5 malaria vaccine reside in intrinsically unstructured n-terminal repetitive sequences. PLoS ONE 2014, 9, e98460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, K.; Arisue, N.; Palacpac, N.M.; Yagi, M.; Tougan, T.; Honma, H.; Ferreira, M.U.; Farnert, A.; Bjorkman, A.; Kaneko, A.; et al. Geographic differentiation of polymorphism in the plasmodium falciparum malaria vaccine candidate gene sera5. Vaccine 2012, 30, 1583–1593. [Google Scholar] [CrossRef]
- Mutalima, N.; Molyneux, E.M.; Johnston, W.T.; Jaffe, H.W.; Kamiza, S.; Borgstein, E.; Mkandawire, N.; Liomba, G.N.; Batumba, M.; Carpenter, L.M.; et al. Impact of infection with human immunodeficiency virus-1 (hiv) on the risk of cancer among children in malawi—Preliminary findings. Infect. Agent Cancer 2010, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, K.; Mita, T.; Jombart, T.; Eriksson, A.; Horibe, S.; Palacpac, N.; Ranford-Cartwright, L.; Sawai, H.; Sakihama, N.; Ohmae, H.; et al. Plasmodium falciparum accompanied the human expansion out of Africa. Curr. Biol. 2010, 20, 1283–1289. [Google Scholar] [CrossRef] [Green Version]
- Librado, P.; Rozas, J. Dnasp v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Nei, M.; Gojobori, T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 1986, 3, 418–426. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. Mega x: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Wright, S. The interpretation of population structure by f-statistics with special regard to systems of mating. Evolution 1965, 19, 395–420. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under linux and windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Sumner, K.M.; Freedman, E.; Abel, L.; Obala, A.; Pence, B.W.; Wesolowski, A.; Meshnick, S.R.; Prudhomme-OߣMeara, W.; Taylor, S.M. Genotyping cognate plasmodium falciparum in humans and mosquitoes to estimate onward transmission of asymptomatic infections. Nat. Commun. 2021, 12, 909. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, A.G.; Sixpence, A.; Chimenya, M.; Damson, M.; Sorkin, J.D.; Wilson, M.L.; Seydel, K.; Hochman, S.; Mathanga, D.P.; Taylor, T.E.; et al. Clinical implications of asymptomatic plasmodium falciparum infections in malawi. Clin. Infect. Dis. 2019, 68, 106–112. [Google Scholar] [PubMed]
- Henning, L.; Schellenberg, D.; Smith, T.; Henning, D.; Alonso, P.; Tanner, M.; Mshinda, H.; Beck, H.P.; Felger, I. A prospective study of plasmodium falciparum multiplicity of infection and morbidity in tanzanian children. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 687–694. [Google Scholar] [CrossRef] [Green Version]
- Eldh, M.; Hammar, U.; Arnot, D.; Beck, H.P.; Garcia, A.; Liljander, A.; Mercereau-Puijalon, O.; Migot-Nabias, F.; Mueller, I.; Ntoumi, F.; et al. Multiplicity of asymptomatic plasmodium falciparum infections and risk of clinical malaria: A systematic review and pooled analysis of individual participant data. J. Infect. Dis. 2020, 221, 775–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langhorne, J.; Ndungu, F.M.; Sponaas, A.M.; Marsh, K. Immunity to malaria: More questions than answers. Nat. Immunol. 2008, 9, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Redmond, L.S.; Ogwang, M.D.; Kerchan, P.; Reynolds, S.J.; Tenge, C.N.; Were, P.A.; Kuremu, R.T.; Masalu, N.; Kawira, E.; Otim, I.; et al. Endemic burkitt lymphoma: A complication of asymptomatic malaria in sub-saharan africa based on published literature and primary data from uganda, tanzania, and kenya. Malar. J. 2020, 19, 239. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Beck, H.P.; Kitua, A.; Mwankusye, S.; Felger, I.; Fraser-Hurt, N.; Irion, A.; Alonso, P.; Teuscher, T.; Tanner, M. Age dependence of the multiplicity of plasmodium falciparum infections and of other malariological indices in an area of high endemicity. Trans. R. Soc. Trop. Med. Hyg. 1999, 93 (Suppl. 1), 15–20. [Google Scholar] [CrossRef] [Green Version]
- Nkhoma, S.C.; Trevino, S.G.; Gorena, K.M.; Nair, S.; Khoswe, S.; Jett, C.; Garcia, R.; Daniel, B.; Dia, A.; Terlouw, D.J.; et al. Co-transmission of related malaria parasite lineages shapes within-host parasite diversity. Cell Host Microbe 2020, 27, 93–103. [Google Scholar] [CrossRef]
- Peprah, S.; Ogwang, M.D.; Kerchan, P.; Reynolds, S.J.; Tenge, C.N.; Were, P.A.; Kuremu, R.T.; Wekesa, W.N.; Sumba, P.O.; Masalu, N.; et al. Risk factors for burkitt lymphoma in east african children and minors: A case-control study in malaria-endemic regions in Uganda, Tanzania and Kenya. Int. J. Cancer 2020, 146, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Lavstsen, T.; Berger, S.S.; Wang, C.W.; Petersen, J.E.; Avril, M.; Brazier, A.J.; Freeth, J.; Jespersen, J.S.; Nielsen, M.A.; et al. Severe malaria is associated with parasite binding to endothelial protein c receptor. Nature 2013, 498, 502–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magallon-Tejada, A.; Machevo, S.; Cistero, P.; Lavstsen, T.; Aide, P.; Rubio, M.; Jimenez, A.; Turner, L.; Valmaseda, A.; Gupta, H.; et al. Cytoadhesion to gc1qr through plasmodium falciparum erythrocyte membrane protein 1 in severe malaria. PLoS Pathog. 2016, 12, e1006011. [Google Scholar] [CrossRef] [Green Version]
- Joergensen, L.; Bengtsson, D.C.; Bengtsson, A.; Ronander, E.; Berger, S.S.; Turner, L.; Dalgaard, M.B.; Cham, G.K.; Victor, M.E.; Lavstsen, T.; et al. Surface co-expression of two different pfemp1 antigens on single plasmodium falciparum-infected erythrocytes facilitates binding to icam1 and pecam1. PLoS Pathog. 2010, 6, e1001083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tougan, T.; Edula, J.R.; Takashima, E.; Morita, M.; Shinohara, M.; Shinohara, A.; Tsuboi, T.; Horii, T. Molecular camouflage of plasmodium falciparum merozoites by binding of host vitronectin to p47 fragment of sera5. Sci. Rep. 2018, 8, 5052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, J.L.; Bluming, A.Z.; Templeton, A.C. Burkittߣs lymphoma and tropical splenomegaly syndrome. Lancet 1971, 2, 317. [Google Scholar] [CrossRef]
- Engwerda, C.R.; Beattie, L.; Amante, F.H. The importance of the spleen in malaria. Trends Parasitol. 2005, 21, 75–80. [Google Scholar] [CrossRef]
- De-The, G.; Geser, A.; Day, N.E.; Tukei, P.M.; Williams, E.H.; Beri, D.P.; Smith, P.G.; Dean, A.G.; Bronkamm, G.W.; Feorino, P.; et al. Epidemiological evidence for causal relationship between epstein-barr virus and burkittߣs lymphoma from ugandan prospective study. Nature 1978, 274, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Nkhoma, S.C.; Banda, R.L.; Khoswe, S.; Dzoole-Mwale, T.J.; Ward, S.A. Intra-host dynamics of co-infecting parasite genotypes in asymptomatic malaria patients. Infect. Genet. Evol. 2018, 65, 414–424. [Google Scholar] [CrossRef] [PubMed]


| Subject Characteristics | Sample Size | Gender | Age Group | Sera5 PCR | Sera5 Sequence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (%) | Missing Age | Mean (SD) | 0–5yrs | 6–10yrs | 11–15yrs * | Negative | Positive | Single | Mixed | Indeterminate † | ||
| Case Status | ||||||||||||
| Cases | 200 | 125 (62.5) | 8 | 7.2 (2.8) | 69 (34.5) | 101 (50.5) | 22 (11.0) | 92 (46.0) | 108 (54.0) | 63 (58.3) | 45 (41.7) | - |
| Controls | 140 | 82 (58.6) | 5 | 6.9 (4.4) | 66 (47.1) | 36 (25.7) | 33 (23.6) | 70 (50.0) | 70 (50.0) | 52 (74.3) | 17 (24.3) | 1 (1.4) |
| Association with eBL | ||||||||||||
| Odds ratio [OR 95% CI] | - | - | - | - | - | - | - | - | - | Ref | 2.18 (1.12, 4.26) | - |
| Adjusted OR ‡ [95% CI] | - | - | - | - | - | - | - | - | - | Ref | 2.40 (1.11, 5.17) | - |
| Diagnose in Controls | ||||||||||||
| Leukemias | 9 | 3 (33.3) | 0 | 7.6 (4.5) | 4 (44.4) | 2 (22.2) | 3 (33.3) | 7 (77.8) | 2 (22.2) | 1 (50.0) | 1 (50.0) | - |
| Lymphomas | 26 | 18 (69.2) | 1 | 10.2 (4.1) | 4 (16.0) | 8 (32.0) | 13 (52.0) | 13 (50.0) | 13 (50.0) | 11 (84.6) | 2 (15.4) | - |
| Neuroblastomas | 8 | 5 (62.5) | 0 | 7.0 (3.9) | 3 (37.5) | 4 (50.0) | 1 (12.5) | 6 (75.0) | 2 (25.0) | 2 (100.0) | 0 (0.0) | - |
| Retinoblastoma | 9 | 3 (33.3) | 0 | 4.0 (2.8) | 8 (88.9) | 0 (0.0) | 1 (11.1) | 5 (55.6) | 4 (44.4) | 2 (50.0) | 2 (50.0) | - |
| Renal tumors | 28 | 21 (75.0) | 0 | 3.6 (2.9) | 24 (85.7) | 3 (10.7) | 1 (3.6) | 8 (28.6) | 20 (71.4) | 15 (75.0) | 5 (25.0) | - |
| Hepatic tumors | 6 | 3 (50.0) | 0 | 7.6 (2.7) | 2 (33.3) | 3 (50.0) | 1 (16.7) | 2 (33.3) | 4 (66.7) | 3 (75.0) | 1 (25.0) | - |
| Bone tumors | 2 | 1 (50.0) | 0 | 10.3 (5.4) | 0 (0.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | 1 (100.0) | 0 (0.0) | - |
| Soft tissue sarcomas | 19 | 14 (73.7) | 1 | 7.8 (4.2) | 8 (44.4) | 5 (27.8) | 5 (27.8) | 11 (57.9) | 8 (42.1) | 6 (75.0) | 2 (25.0) | - |
| Germ cell tumors | 8 | 3 (37.5) | 0 | 6.8 (4.0) | 4 (50.0) | 2 (25.0) | 2 (25.0) | 3 (37.5) | 5 (62.5) | 3 (60.0) | 2 (40.0) | - |
| Epithelial tumors | 2 | 0 (0.0) | 0 | 6.7 (7.8) | 1 (50.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | 1 (100.0) | 0 (0.0) | - |
| Other tumors | 1 | 0 (0.0) | 0 | 13.5 (-) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Non-malignancies | 10 | 6 (60.0) | 0 | 7.3 (4.7) | 3 (30.0) | 5 (50.0) | 2 (20.0) | 5 (50.0) | 5 (50.0) | 4 (80.0) | 0 (0.0) | 1 (20.0) |
| Not well specified | 12 | 5 (41.7) | 3 | 6.2 (4.6) | 5 (55.6) | 3 (33.3) | 1 (11.1) | 7 (58.3) | 5 (41.7) | 3 (60.0) | 2 (40.0) | - |
| Country | n | Entire Sequence | OctR Region | SerR Region | NonR 2562 bp Region | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Haplotypes | Hd ± SD | No. of Haplotypes | Hd ± SD | No. of Haplotypes | Hd ± SD | No. of Haplotypes | Hd ± SD | ||
| Malawi and Mozambique | 115 | 96 (88) | 0.994 ± 0.003 | 34 (34) | 0.809 ± 0.032 | 42 (39) | 0.935 ± 0.011 | 26 (16) | 0.545 ± 0.011 |
| eBL case | 63 | 54 (50) | 0.993 ± 0.005 | 19 (19) | 0.786 ± 0.047 | 28 (27) | 0.928 ± 0.019 | 16 (9) | 0.551 ± 0.075 |
| Control | 52 | 47 (45) | 0.996 ± 0.002 | 21 (21) | 0.839 ± 0.040 | 26 (23) | 0.945 ± 0.016 | 14 (9) | 0.544 ± 0.083 |
| Tanzania | 55 | 44 (42) | 0.991 ± 0.006 | 21(21) | 0.804 ± 0.054 | 30 (30) | 0.968 ± 0.010 | 11 (9) | 0.362 ± 0.084 |
| Ghana | 33 | 31 (29) | 0.996 ± 0.009 | 16 (16) | 0.873 ± 0.048 | 23 (23) | 0.968 ± 0.018 | 8 (7) | 0.472 ± 0.106 |
| Country | n | No. of Polynmorphic Sites | No. of Substitutions | Nucleotide Diversity | Codon-Based Evolutionary Divergence | |||
|---|---|---|---|---|---|---|---|---|
| Synonymous | Non-Synonymous | π ± SD | dS ± SE | dN ± SE | p Value | |||
| Malawi and Mozambique | 115 | 29 | 12 | 17 | 0.00029 ± 0.00004 | 0.00050 ± 0.00012 | 0.00024 ± 0.00008 | dS > dN: 0.0595 |
| eBL case | 63 | 17 | 8 | 9 | 0.00028 ± 0.00005 | 0.00055 ± 0.00017 | 0.00021 ± 0.00009 | dS > dN: 0.0450 |
| Control | 52 | 16 | 6 | 10 | 0.00031 ± 0.00006 | 0.00044 ± 0.00017 | 0.00027 ± 0.00011 | dS > dN: 0.1943 |
| Tanzania | 55 | 11 | 1 | 10 | 0.00021 ± 0.00007 | 0.00007 ± 0.00007 | 0.00025 ± 0.00008 | dN > dS: 0.0549 |
| Ghana | 33 | 10 | 1 | 10 | 0.00032 ± 0.00011 | 0.00000 ± 0.00000 | 0.00040 ± 0.00015 | dN > dS: 0.0034 |
| (A) Non-synonymous change. | |||||||||||||||||||||||||||
| Nucleotide Position | 3 8 3 | 4 7 5 | 5 6 2 | 5 6 6 | 5 7 1 | 7 6 7 | 7 8 2 | 7 9 2 | 9 8 8 | 1 3 0 4 | 1 5 1 6 | 1 5 2 4 | 1 6 1 3 | 1 7 2 1 | 1 8 9 2 | 1 9 6 4 | 2 0 3 8 | 2 0 6 5 | 2 1 5 5 | 2 2 6 9 | 2 3 2 5 | 2 3 2 6 | 2 4 4 9 | 2 4 9 1 | 2 6 7 8 | 2 8 0 1 | 2 8 3 3 |
| Common | G | K | G | A | G | G | G | G | A | A | G | G | A | G | A | T | A | T | G | G | C | G | G | A | A | G | A |
| Variation | C | G | A | C | A | A | A | T | T | G | C | T | T | A | G | C | G | G | A | A | A | A | T | G | G | A | G |
| Amino Acid Position | 1 2 8 | 1 5 9 | 1 8 8 | 1 8 9 | 1 9 1 | 2 5 6 | 2 6 1 | 2 6 4 | 3 3 0 | 4 3 5 | 5 0 6 | 5 0 8 | 5 3 8 | 5 7 4 | 6 3 1 | 6 5 5 | 6 8 0 | 6 8 9 | 7 1 9 | 7 5 7 | 7 7 5 | 7 7 6 | 8 1 7 | 8 3 1 | 8 9 3 | 9 3 4 | 9 4 5 |
| Common | C | K | G | E | G | C | N | L | I | D | V | K | D | R | E | L | M | L | V | D | S | E | V | T | Q | R | K |
| Variation | S | E | S | A | S | Y | S | F | L | G | L | N | V | K | G | S | V | V | I | N | R | K | F | A | R | H | E |
| Malawi and Mozambique, n = 115 | 1 | - | - | - | 1 | 1 | - | - | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 | 12 | - | 1 | 1 | - | 2 | 1 | 1 | 1 | - | - |
| eBL case, n = 63 | 1 | - | - | - | 1 | - | - | - | - | 1 | 1 | - | - | - | - | 1 | 6 | - | - | 1 | 1 | 1 | - | - | |||
| Control, n = 52 | - | - | - | - | - | 1 | - | - | 1 | - | - | 1 | - | 1 | 1 | - | 6 | - | 1 | 1 | - | 1 | 1 | - | - | - | - |
| Tanzania, n = 55 | - | 1 | 1 | - | 2 | - | 1 | - | 1 | - | - | - | 2 | - | - | - | 3 | - | - | - | 1 | - | - | - | - | 1 | 1 |
| Ghana, n = 33 | - | 1 | 1 | 1 | 1 | - | - | 1 | 1 | - | - | - | - | - | - | - | 4 | 1 | - | - | - | - | - | - | - | 1 | 1 |
| (B) Synonymous change. | |||||||||||||||||||||||||||
| Nucleotide Position | 5 6 4 | 7 7 7 | 7 9 2 | 9 0 3 | 1 2 2 1 | 1 5 9 0 | 1 6 0 2 | 1 7 4 9 | 1 9 2 3 | 2 1 0 6 | 2 3 4 6 | 2 8 8 2 | 2 6 3 1 | ||||||||||||||
| Common | T | A | T | A | T | T | A | T | T | A | T | T | A | ||||||||||||||
| Variation | G | G | C | G | C | C | G | G | C | G | C | C | G | ||||||||||||||
| Amino Acid Position | 1 8 8 | 2 5 9 | 2 6 4 | 3 0 1 | 4 0 7 | 5 3 0 | 5 3 4 | 5 8 3 | 6 4 1 | 7 0 2 | 7 8 2 | 7 9 4 | 8 7 7 | ||||||||||||||
| Amino Acid | G | G | L | E | S | D | P | S | S | G | Y | G | L | ||||||||||||||
| Malawi and Mozambique, n = 115 | 2 | 1 | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | ||||||||||||||
| eBL Case, n = 63 | 2 | 1 | - | 1 | 1 | 1 | - | 1 | - | - | 1 | 1 | - | ||||||||||||||
| Control, n = 52 | - | - | - | - | - | - | 1 | - | 1 | 1 | 1 | 1 | 1 | ||||||||||||||
| Tanzania, n = 55 | - | - | - | - | - | - | - | - | - | - | 1 | - | - | ||||||||||||||
| Ghana, n = 33 | - | - | 1 | - | - | - | - | - | - | - | - | - | - | ||||||||||||||
| P. falciparum sera5Region/Country | Subject Type | Malawi and Mozambique | Tanzania | Ghana | ||
|---|---|---|---|---|---|---|
| All | eBL Case | Control | All | All | ||
| (A) Octamer repeat region | ||||||
| Malawi and Mozambique | Combined | - | 0.9910 | 0.9910 | 0.0721 | 0.0360 |
| eBL case | −0.0094 | - | 0.4595 | 0.1171 | 0.0090 | |
| Control | −0.0097 | −0.0030 | - | 0.0811 | 0.0270 | |
| Tanzania | All subjects | 0.0113 | 0.0085 | 0.0130 | - | 0.0811 |
| Ghana | All subjects | 0.0291 | 0.0271 | 0.0286 | 0.0104 | - |
| (B) Serine repeat region | ||||||
| Malawi and Mozambique | Combined | - | 0.9910 | 0.9910 | 0.0811 | 0.0000 |
| eBL case | −0.0093 | - | 0.6216 | 0.0541 | 0.0000 | |
| Control | −0.0096 | −0.0027 | - | 0.3604 | 0.0451 | |
| Tanzania | All subjects | 0.0069 | 0.0100 | 0.0013 | - | 0.2072 |
| Ghana | All subjects | 0.0202 | 0.0204 | 0.0181 | 0.0039 | - |
| (C) Non-repeat 2562 bp sequence | ||||||
| Malawi and Mozambique | Combined | - | 0.9910 | 0.9910 | 0.2973 | 0.5676 |
| eBL case | −0.0099 | - | 0.9279 | 0.4234 | 0.5946 | |
| Control | −0.0105 | −0.0056 | - | 0.2342 | 0.8018 | |
| Tanzania | All subjects | 0.0011 | −0.0002 | 0.0023 | - | 0.5586 |
| Ghana | All subjects | −0.0033 | −0.0037 | −0.0067 | −0.0044 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arisue, N.; Chagaluka, G.; Palacpac, N.M.Q.; Johnston, W.T.; Mutalima, N.; Peprah, S.; Bhatia, K.; Borgstein, E.; Liomba, G.N.; Kamiza, S.; et al. Assessment of Mixed Plasmodium falciparum sera5 Infection in Endemic Burkitt Lymphoma: A Case-Control Study in Malawi. Cancers 2021, 13, 1692. https://doi.org/10.3390/cancers13071692
Arisue N, Chagaluka G, Palacpac NMQ, Johnston WT, Mutalima N, Peprah S, Bhatia K, Borgstein E, Liomba GN, Kamiza S, et al. Assessment of Mixed Plasmodium falciparum sera5 Infection in Endemic Burkitt Lymphoma: A Case-Control Study in Malawi. Cancers. 2021; 13(7):1692. https://doi.org/10.3390/cancers13071692
Chicago/Turabian StyleArisue, Nobuko, George Chagaluka, Nirianne Marie Q. Palacpac, W. Thomas Johnston, Nora Mutalima, Sally Peprah, Kishor Bhatia, Eric Borgstein, George N. Liomba, Steve Kamiza, and et al. 2021. "Assessment of Mixed Plasmodium falciparum sera5 Infection in Endemic Burkitt Lymphoma: A Case-Control Study in Malawi" Cancers 13, no. 7: 1692. https://doi.org/10.3390/cancers13071692
APA StyleArisue, N., Chagaluka, G., Palacpac, N. M. Q., Johnston, W. T., Mutalima, N., Peprah, S., Bhatia, K., Borgstein, E., Liomba, G. N., Kamiza, S., Mkandawire, N., Mitambo, C., Goedert, J. J., Molyneux, E. M., Newton, R., Horii, T., & Mbulaiteye, S. M. (2021). Assessment of Mixed Plasmodium falciparum sera5 Infection in Endemic Burkitt Lymphoma: A Case-Control Study in Malawi. Cancers, 13(7), 1692. https://doi.org/10.3390/cancers13071692