Discordance of KRAS Mutational Status between Primary Tumors and Liver Metastases in Colorectal Cancer: Impact on Long-Term Survival Following Radical Resection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Preoperative Assessment
2.3. Preoperative Chemotherapy
2.4. Surgical Procedure
2.5. KRAS Mutation Analysis
2.6. Primary Outcome
2.7. Secondary Outcome
2.8. Statistical Analysis
3. Results
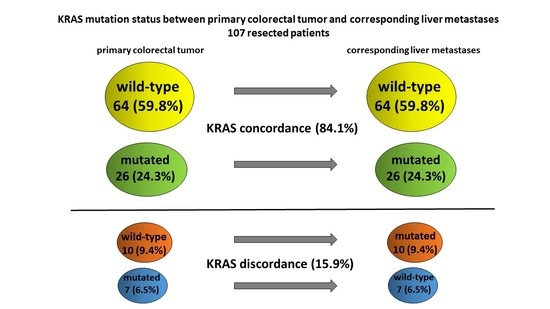
3.1. KRAS Mutation Analysis
3.1.1. Incidence of KRAS Mutation
3.1.2. Incidence of KRAS Discordance
3.1.3. KRAS Discordance and Preoperative Chemotherapy
3.2. Overall Survival
3.3. Predictors of KRAS Discordance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- House, M.G.; Ito, H.; Gonen, M.; Fong, Y.; Allen, P.J.; DeMatteo, R.P.; Brennan, M.F.; Blumgart, L.H.; Jarnagin, W.R.; D’Angelica, M.I. Survival after hepatic resection for metastatic colorectal cancer: Trends in outcomes for 1600 patients during two decades at a single institution. J. Am. Coll. Surg. 2010, 210, 744–752. [Google Scholar] [CrossRef]
- Giuliante, F.; Ardito, F.; Vellone, M.; Ranucci, G.; Federico, B.; Giovannini, I.; Nuzzo, G. Role of the surgeon as a variable in long-term survival after liver resection for colorectal metastases. J. Surg. Oncol. 2009, 100, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.C.; Primrose, J.N.; Colquitt, J.L.; Garden, O.J.; Poston, G.J.; Rees, M. Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies. Br. J. Cancer 2006, 94, 982–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, M.; Tekkis, P.P.; Welsh, F.K.; O’Rourke, T.; John, T.G. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann. Surg. 2008, 247, 125–135. [Google Scholar] [CrossRef] [PubMed]
- de Haas, R.J.; Wicherts, D.A.; Andreani, P.; Pascal, G.; Saliba, F.; Ichai, P.; Adam, R.; Castaing, D.; Azoulay, D. Impact of expanding criteria for resectability of colorectal metastases on short- and long-term outcomes after hepatic resection. Ann. Surg. 2011, 253, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Chun, Y.S.; Kopetz, S.E.; Vauthey, J.N. Biomarkers in colorectal liver metastases. Br. J. Surg. 2018, 105, 618–627. [Google Scholar] [CrossRef]
- Brudvik, K.W.; Jones, R.P.; Giuliante, F.; Shindoh, J.; Passot, G.; Chung, M.H.; Song, J.; Li, L.; Dagenborg, V.J.; Fretland, Å.A.; et al. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann. Surg. 2019, 269, 120–126. [Google Scholar] [CrossRef]
- Oliveira, C.; Westra, J.L.; Arango, D.; Ollikainen, M.; Domingo, E.; Ferreira, A.; Velho, S.; Niessen, R.; Lagerstedt, K.; Alhopuro, P.; et al. Distinct patterns of KRAS mutations in colorectal carcinomas according to germline mismatch repair defects and hMLH1 methylation status. Hum. Mol. Genet. 2004, 13, 2303–2311. [Google Scholar] [CrossRef]
- Vauthey, J.N.; Zimmitti, G.; Kopetz, S.E.; Shindoh, J.; Chen, S.S.; Andreou, A.; Curley, S.A.; Aloia, T.A.; Maru, D.M. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann. Surg. 2013, 258, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Kemeny, N.E.; Chou, J.F.; Capanu, M.; Gewirtz, A.N.; Cercek, A.; Kingham, T.P.; Jarnagin, W.R.; Fong, Y.C.; DeMatteo, R.P.; Allen, P.J.; et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer 2014, 120, 3965–3971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zauber, P.; Sabbath-Solitare, M.; Marotta, S.P.; Bishop, D.T. Molecular changes in the Ki-ras and APC genes in primary colorectal carcinoma and synchronous metastases compared with the findings in accompanying adenomas. Mol. Pathol. 2003, 56, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Etienne-Grimaldi, M.C.; Formento, J.L.; Francoual, M.; François, E.; Formento, P.; Renée, N.; Laurent-Puig, P.; Chazal, M.; Benchimol, D.; Delpero, J.R.; et al. K-Ras mutations and treatment outcome in colorectal cancer patients receiving exclusive fluoropyrimidine therapy. Clin. Cancer Res. 2008, 14, 4830–4835. [Google Scholar] [CrossRef] [Green Version]
- Artale, S.; Sartore-Bianchi, A.; Veronese, S.M.; Gambi, V.; Sarnataro, C.S.; Gambacorta, M.; Lauricella, C.; Siena, S. Mutations of KRAS and BRAF in primary and matched metastatic sites of colorectal cancer. J. Clin. Oncol. 2008, 26, 4217–4219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santini, D.; Loupakis, F.; Vincenzi, B.; Floriani, I.; Stasi, I.; Canestrari, E.; Rulli, E.; Maltese, P.E.; Andreoni, F.; Masi, G.; et al. High concordance of KRAS status between primary colorectal tumors and related metastatic sites: Implications for clinical practice. Oncologist 2008, 13, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Molinari, F.; Martin, V.; Saletti, P.; De Dosso, S.; Spitale, A.; Camponovo, A.; Bordoni, A.; Crippa, S.; Mazzucchelli, L.; Frattini, M. Differing deregulation of EGFR and downstream proteins in primary colorectal cancer and related metastatic sites may be clinically relevant. Br. J. Cancer 2009, 100, 1087–1094. [Google Scholar] [CrossRef]
- Knijn, N.; Mekenkamp, L.J.; Klomp, M.; Vink-Börger, M.E.; Tol, J.; Teerenstra, S.; Meijer, J.W.; Tebar, M.; Riemersma, S.; van Krieken, J.H.; et al. KRAS mutation analysis: A comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br. J. Cancer 2011, 104, 1020–1026. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.Z.; Bai, L.; Wang, F.; Zhang, Z.C.; Wang, F.; Zeng, Z.L.; Zeng, J.B.; Zhang, D.S.; Wang, F.H.; Wang, Z.Q.; et al. Comparison of KRAS mutation status between primary tumor and metastasis in Chinese colorectal cancer patients. Med. Oncol. 2016, 33, 71. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, D.S.; Barriuso, J.; Mullamitha, S.; Saunders, M.P.; O’Dwyer, S.T.; Aziz, O. Biomarker concordance between primary colorectal cancer and its metastases. EBioMedicine 2019, 40, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Dupré, A.; Malik, H.Z.; Jones, R.P.; Diaz-Nieto, R.; Fenwick, S.W.; Poston, G.J. Influence of the primary tumour location in patients undergoing surgery for colorectal liver metastases. Eur. J. Surg. Oncol. 2018, 44, 80–86. [Google Scholar] [CrossRef]
- Goffredo, P.; Utria, A.F.; Beck, A.C.; Chun, Y.S.; Howe, J.R.; Weigel, R.J.; Vauthey, J.N.; Hassan, I. The Prognostic Impact of KRAS Mutation in Patients Having Curative Resection of Synchronous Colorectal Liver Metastases. J. Gastrointest. Surg. 2019, 23, 1957–1963. [Google Scholar] [CrossRef]
- Ardito, F.; Vellone, M.; Cassano, A.; De Rose, A.M.; Pozzo, C.; Coppola, A.; Federico, B.; Giovannini, I.; Barone, C.; Nuzzo, G.; et al. Chance of cure following liver resection for initially unresectable colorectal metastases: Analysis of actual 5-year survival. J. Gastrointest. Surg. 2013, 17, 352–359. [Google Scholar] [CrossRef]
- Ardito, F.; Vellone, M.; Barbaro, B.; Grande, G.; Clemente, G.; Giovannini, I.; Federico, B.; Bonomo, L.; Nuzzo, G.; Giuliante, F. Right and extended-right hepatectomies for unilobar colorectal metastases: Impact of portal vein embolization on long-term outcome and liver recurrence. Surgery 2013, 153, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Panettieri, E.; Vellone, M.; Ferrucci, M.; Coppola, A.; Silvestrini, N.; Arena, V.; Adducci, E.; Capelli, G.; Vecchio, F.M.; et al. The impact of R1 resection for colorectal liver metastases on local recurrence and overall survival in the era of modern chemotherapy: An analysis of 1,428 resection areas. Surgery 2019, 165, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Belghiti, J.; Clavien, P.A. Terminology committee of the IHPBA. Terminology of liver anatomy and resections. HPB Surg. 2000, 2, 333–339. [Google Scholar] [CrossRef]
- Giuliante, F.; Nuzzo, G.; Ardito, F.; Vellone, M.; De Cosmo, G.; Giovannini, I. Extraparenchymal control of hepatic veins during mesohepatectomy. J. Am. Coll. Surg. 2008, 206, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.; Strippoli, A.; Orlandi, A.; Martini, M.; Calegari, M.A.; Schinzari, G.; Di Salvatore, M.; Cenci, T.; Cassano, A.; Larocca, L.M.; et al. KRAS mutational status affects oxaliplatin-based chemotherapy independently from basal mRNA ERCC-1 expression in metastatic colorectal cancer patients. Br. J. Cancer 2013, 108, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natalicchio, M.I.; Improta, G.; Zupa, A.; Cursio, O.E.; Stampone, E.; Possidente, L.; Teresa Gerardi, A.M.; Vita, G.; Martini, M.; Cassano, A.; et al. Pyrosequencing evaluation of low-frequency KRAS mutant alleles for EGF receptor therapy selection in metastatic colorectal carcinoma. Future Oncol. 2014, 10, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, A.; Cocomazzi, A.; Basso, M.; Cenci, T.; Ricci, R.; Pierconti, F.; Cassano, A.; Fiorentino, V.; Barone, C.; Bria, E.; et al. c-MYC Expression Is a Possible Keystone in the Colorectal Cancer Resistance to EGFR Inhibitors. Cancers 2020, 12, 638. [Google Scholar] [CrossRef] [Green Version]
- De Roock, W.; De Vriendt, V.; Normanno, N.; Ciardiello, F.; Tejpar, S. KRAS, BRAF, PIK3CA, and PTEN mutations: Implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011, 12, 594–603. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Jones, R.P.; Brudvik, K.W.; Franklin, J.M.; Poston, G.J. Precision surgery for colorectal liver metastases: Opportunities and challenges of omics-based decision making. Eur. J. Surg. Oncol. 2017, 43, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Sayagués, J.M.; Abad Mdel, M.; Melchor, H.B.; Gutiérrez, M.L.; González-González, M.; Jensen, E.; Bengoechea, O.; Fonseca, E.; Orfao, A.; Muñoz-Bellvis, L. Intratumoural cytogenetic heterogeneity of sporadic colorectal carcinomas suggests several pathways to liver metastasis. J. Pathol. 2010, 221, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen, S.; Sayagués, J.M.; Bengoechea, O.; Anduaga, M.F.; Alcazar, J.A.; Gervas, R.; García, J.; Orfao, A.; Bellvis, L.M.; Sarasquete, M.E.; et al. Spatio-temporal tumor heterogeneity in metastatic CRC tumors: A mutational-based approach. Oncotarget 2018, 9, 34279–34288. [Google Scholar] [CrossRef]
- Bouchahda, M.; Karaboué, A.; Saffroy, R.; Innominato, P.; Gorden, L.; Guettier, C.; Adam, R.; Lévi, F. Acquired KRAS mutations during progression of colorectal cancer metastases: Possible implications for therapy and prognosis. Cancer Chemother. Pharmacol. 2010, 66, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.A.; Pugh, S.A.; Maishman, T.; Eminton, Z.; Mellor, J.; Whitehead, A.; Stanton, L.; Radford, M.; Corkhill, A.; Griffiths, G.O.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): Long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 398–411. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef] [Green Version]
- Baretti, M.; Personeni, N.; Destro, A.; Santoro, A.; Rimassa, L. Emergence of KRAS-mutation in liver metastases after an anti-EGFR treatment in patient with colorectal cancer: Are we aware of the therapeutic impact of intratumor heterogeneity? Cancer Biol. Ther. 2018, 19, 659–663. [Google Scholar] [CrossRef]
- Siravegna, G.; Mussolin, B.; Buscarino, M.; Corti, G.; Cassingena, A.; Crisafulli, G.; Ponzetti, A.; Cremolini, C.; Amatu, A.; Lauricella, C. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 2015, 21, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Van Emburgh, B.O.; Arena, S.; Siravegna, G.; Lazzari, L.; Crisafulli, G.; Corti, G.; Mussolin, B.; Baldi, F.; Buscarino, M.; Bartolini, A.; et al. Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nat. Commun. 2016, 7, 13665. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.M.; Montagut, C.; Wainberg, Z.A.; Ronga, P.; Audhuy, F.; Taieb, J.; Stintzing, S.; Siena, S.; Santini, D. Optimising the use of cetuximab in the continuum of care for patients with metastatic colorectal cancer. ESMO Open 2018, 3, e000353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremolini, C.; Rossini, D.; Dell’Aquila, E.; Lonardi, S.; Conca, E.; Del Re, M.; Busico, A.; Pietrantonio, F.; Danesi, R.; Aprile, G.; et al. Rechallenge for Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer With Acquired Resistance to First-line Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variable | No. (%) |
|---|---|
| Age, median (IQR) | 63 (32–83) |
| Gender | |
| Male | 67 (62.6) |
| Female | 40 (37.4) |
| Primary tumor | |
| Location | |
| Right-sided | 29 (27.1) |
| Left-sided | 46 (43.0) |
| Rectum | 32 (29.9) |
| N stage | |
| N0 | 31 (29.0) |
| N1 | 76 (71.0) |
| Liver metastases | |
| Timing of diagnosis | |
| Synchronous | 71 (66.3) |
| Metachronous | 36 (33.7) |
| Largest size (cm) | |
| <5 cm | 87 (81.3) |
| ≥5 cm | 20 (18.7) |
| No. of metastases | |
| ≤3 metastases | 68 (63.5) |
| >3 metastases | 39 (36.5) |
| Distribution | |
| Unilobar | 54 (50.5) |
| Bilobar | 53 (49.5) |
| Initial resectability | |
| Resectable | 93 (86.9) |
| Unresectable | 14 (13.1) |
| Preoperative chemotherapy | 80 (74.8) |
| Oxaliplatin-based | 43 (53.75) |
| Irinotecan-based | 28 (35.0) |
| Both | 5 (6.25) |
| Other | 4 (5.0) |
| No. of cycles | |
| ≤6 | 24 (30.0) |
| >6 | 56 (70.0) |
| No. of lines | |
| 1 | 74 (92.5) |
| >1 | 6 (7.5) |
| Associated targeting agents | 59/80 (73.7) |
| Bevacizumab | 33/59 (55.9) |
| Cetuximab | 26/59 (44.1) |
| Radiological clinical response | |
| Partial | 58 (72.5) |
| Stabilization | 12 (15.0) |
| Progression | 10 (12.5) |
| Operative features | |
| Major hepatectomy | |
| Yes | 29 (27.1) |
| No | 78 (72.9) |
| Radicality of liver resection | |
| R0 | 75 (70.1) |
| R1 | 32 (29.9) |
| KRAS Mutation Analysis | Primary Tumor, No. (%) | CRLM, No. (%) | p |
|---|---|---|---|
| KRAS mutation | 33 (30.8) | 36 (33.6) | |
| Codon 12 | 16/33 (48.5) | 21/36 (58.3%) | 0.412 |
| p.(Gly12Val) | 6 | 7 | |
| p.(Gly12Asp) | 4 | 7 | |
| p.(Gly12Ser) | 2 | 2 | |
| p.(Gly12Cys) | 2 | 2 | |
| p.(Gly12Ala) | 2 | 2 | |
| p.(G12R/S/C) | - | 1 | |
| Codon 13 | 7/33 (21.2) | 7/36 (19.4) | 0.855 |
| p.(Gly13Asp) | 7 | 7 | |
| p.(Ala146Thr) | 6/33 (18.1) | 5/36 (13.9) | 0.626 |
| p.(Gln22Lys) | 2/33 (6.1) | 2/36 (5.6) | 0.928 |
| p.(Ala59Xaa) | - | 1/36 (2.8) | |
| p.(Gln61Xaa) | 2/33 (6.1) |
| Variable | No. (%) | 5-Year OS (%) | Univariate Analysis | Multivariable Analysis | |
|---|---|---|---|---|---|
| p Value | HR (95%CI) | p | |||
| Age (years) | |||||
| <70 | 79 (73.8) | 61.3 | 0.365 | ||
| ≥70 | 28 (26.2) | 41.7 | |||
| Gender | |||||
| Male | 67 (62.6) | 60.5 | 0.359 | ||
| Female | 40 (37.4) | 48.3 | |||
| Primary tumor | |||||
| Location | |||||
| Right-sided | 29 (27.1) | 36.1 | 0.048 | ||
| Left-sided | 46 (43.0) | 49.8 | |||
| Rectum | 32 (29.9) | 63.1 | |||
| N stage | |||||
| N0 | 31 (29.0) | 63.0 | 0.338 | ||
| N1 | 76 (71.0) | 52.2 | |||
| Liver metastases | |||||
| Timing of diagnosis | |||||
| Synchronous | 71 (66.3) | 50.3 | 0.073 | ||
| Metachronous | 36 (33.7) | 63.4 | |||
| Largest size (cm) | |||||
| <5 | 87 (81.3) | 59.2 | 0.201 | ||
| ≥5 | 20 (18.7) | 38.0 | |||
| No. of metastases | |||||
| ≤3 | 68 (63.5) | 53.7 | 0.014 | ||
| >3 | 39 (36.5) | 40.8 | |||
| Distribution | |||||
| Unilobar | 54 (50.5) | 69.2 | 0.001 | 2.550 (1.188–5.475) | 0.016 |
| Bilobar | 53 (49.5) | 38.9 | |||
| Initial resectability | |||||
| Resectable | 93 (86.9) | 60.0 | <0.001 | 3.317 (1.297–8.483) | 0.012 |
| Unresectable | 14 (13.1) | 28.8 | |||
| Preoperative chemotherapy | |||||
| Yes | 80 (74.8) | 54.8 | 0.339 | ||
| No | 27 (25.2) | 58.0 | |||
| Irinotecan-based | 28/80 (35.0) | 60.1 | 0.816 | ||
| Oxaliplatin-based | 43/80 (53.75) | 51.0 | |||
| Targeting agents | |||||
| Yes | 59/80 (73.75) | 53.3 | 0.270 | ||
| No | 21/80 (26.25) | 56.9 | |||
| No. of cycles | |||||
| ≤6 | 24 (30.0) | 68.4 | 0.241 | ||
| >6 | 56 (70.0) | 50.0 | |||
| No. of lines | |||||
| 1 | 74 (92.5) | 55.5 | 0.929 | ||
| >1 | 6 (7.5) | 0 | |||
| Radiological clinical response | |||||
| Partial/stabilization | 70 (87.5) | 56.8 | 0.434 | ||
| Progression | 10 (12.5) | 30.0 | |||
| Operative features | |||||
| Major hepatectomy | |||||
| Yes | 29 (27.1) | 48.0 | 0.169 | ||
| No | 78 (72.9) | 58.8 | |||
| Radicality of resection | |||||
| R0 | 75 (70.1) | 69.4 | <0.001 | 2.709 (1.452–5.056) | 0.002 |
| R1 | 32 (29.9) | 30.8 | |||
| KRAS mutation status | 0.020 | 0.002 | |||
| wild-type in primary tumor and CRLM | 64 (59.8) | 63.6 | |||
| mutated in primary tumor and CRLM | 26 (24.3) | 38.0 | 1.889 (0.746–4.786) | 0.180 | |
| wild-type in primary tumor, mutated in CRLM | 10 (9.4) | 25.0 | 6.332 (2.398–16.715) | <0.001 | |
| mutated in primary tumor, wild-type in CRLM | 7 (6.5) | 60.0 | 1.467 (0.320–6.723) | 0.622 |
| Variable | Univariable Analysis p | Multivariable Analysis OR (95% CI) | p |
|---|---|---|---|
| Age (years) ≥70 | 0.018 | ||
| Male sex | 0.391 | ||
| Primary tumor location | |||
| Right-sided | 0.744 | ||
| Left-sided | 0.780 | ||
| Rectum | 0.469 | ||
| Positive lymph nodes in primary tumor | 0.775 | ||
| Synchronous CRLM | 0.798 | ||
| CRLM size ≥5 cm | 0.998 | ||
| >3 CRLM | 0.155 | 4.600 (1.020–20.734) | 0.047 |
| Bilobar CRLM | 0.206 | ||
| Initially unresectable CRLM | 0.746 | ||
| Administration of preoperative chemotherapy | 0.268 | ||
| Oxaliplatin-based chemotherapy | 0.655 | ||
| Irinotecan-based chemotherapy | 0.167 | ||
| Association of targeting agents | 0.033 | ||
| Association of Bevacizumab | 0.167 | 0.072 (0.007–0.716) | 0.025 |
| Association of Cetuximab | 0.291 | ||
| >6 cycles of chemotherapy | 0.280 | ||
| Progression after chemotherapy | 0.133 | ||
| Major hepatectomy | 0.598 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardito, F.; Razionale, F.; Salvatore, L.; Cenci, T.; Vellone, M.; Basso, M.; Panettieri, E.; Calegari, M.A.; Tortora, G.; Martini, M.; et al. Discordance of KRAS Mutational Status between Primary Tumors and Liver Metastases in Colorectal Cancer: Impact on Long-Term Survival Following Radical Resection. Cancers 2021, 13, 2148. https://doi.org/10.3390/cancers13092148
Ardito F, Razionale F, Salvatore L, Cenci T, Vellone M, Basso M, Panettieri E, Calegari MA, Tortora G, Martini M, et al. Discordance of KRAS Mutational Status between Primary Tumors and Liver Metastases in Colorectal Cancer: Impact on Long-Term Survival Following Radical Resection. Cancers. 2021; 13(9):2148. https://doi.org/10.3390/cancers13092148
Chicago/Turabian StyleArdito, Francesco, Francesco Razionale, Lisa Salvatore, Tonia Cenci, Maria Vellone, Michele Basso, Elena Panettieri, Maria Alessandra Calegari, Giampaolo Tortora, Maurizio Martini, and et al. 2021. "Discordance of KRAS Mutational Status between Primary Tumors and Liver Metastases in Colorectal Cancer: Impact on Long-Term Survival Following Radical Resection" Cancers 13, no. 9: 2148. https://doi.org/10.3390/cancers13092148
APA StyleArdito, F., Razionale, F., Salvatore, L., Cenci, T., Vellone, M., Basso, M., Panettieri, E., Calegari, M. A., Tortora, G., Martini, M., & Giuliante, F. (2021). Discordance of KRAS Mutational Status between Primary Tumors and Liver Metastases in Colorectal Cancer: Impact on Long-Term Survival Following Radical Resection. Cancers, 13(9), 2148. https://doi.org/10.3390/cancers13092148