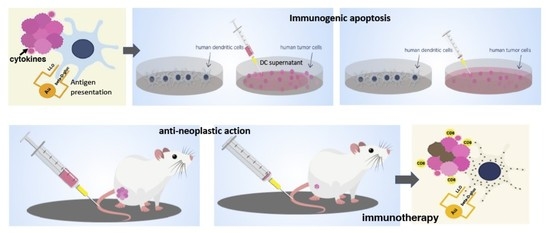
Gold Glyconanoparticles Combined with 91–99 Peptide of the Bacterial Toxin, Listeriolysin O, Are Efficient Immunotherapies in Experimental Bladder Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Mice
2.2. Patients
2.3. Preparation of GNP-LLO91–99 Nanovaccines
2.4. Isolation of MoDCs from Healthy Donors and Patients and Preparation of Murine DCs
2.5. Direct and Immunogenic Apoptosis of Melanoma and Bladder Tumor Cells
2.6. Bladder Tumor Auto Transplants Followed by GNP-LLO91–99 and ICI Immunotherapies
2.7. FACS Analysis and Cytokines
2.8. Statistical Analysis
3. Results and Discussion
3.1. GNP-LLO91–99 Nanovaccines Showed No Toxicity in Human MoDCs or Mice
3.2. GNP-LLO91–99 Nanovaccines Served as Adjuvants for Human MoDCs of Oncologic Patients
3.3. GNP-LLO91–99 Nanovaccines Induced Immunogenic Apoptosis in Bladder Tumors
3.4. Mechanisms of Anti-Neoplastic Abilities of GNP-LLO91–99 Nanovaccines in Mice Models of Melanoma and BC
3.5. GNP-LLO91–99 Nanovaccines Are Effective Immunotherapies That Blocked Immunosuppression
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Cheng, J.; Gu, Y.-J.; Cheng, S.H.; Wong, W.-T. Surface functionalized Gold Nanoparticles for Drug Delivery. J. Biomed. Nanotechnol. 2013, 9, 1362–1369. [Google Scholar] [CrossRef]
- Marradi, M.; Chiodo, F.; García, I.; Penadés, S. Glyconanoparticles as multifunctional and multimodal carbohydrate systems. Chem. Soc. Rev. 2013, 42, 4728–4745. [Google Scholar] [CrossRef]
- Calderon-Gonzalez, R.; Marradi, M.; Garcia, I.; Petrovsky, N.; Alvarez-Dominguez, C. Novel nanoparticle vaccines for listeriosis. Hum. Vaccines Immunother. 2015, 11, 2501–2503. [Google Scholar] [CrossRef] [Green Version]
- Calderon-Gonzalez, R.; Terán-Navarro, H.; García, I.; Marradi, M.; Salcines-Cuevas, D.; Yañez-Diaz, S.; Solis-Angulo, A.; Frande-Cabanes, E.; Fariñas, M.C.; Garcia-Castaño, A.; et al. Gold glyconanoparticles coupled to listeriolysin O 91–99 peptide serve as adjuvant therapy against melanoma. Nanoscale 2017, 9, 10721. [Google Scholar] [CrossRef]
- Calderon-Gonzalez1, R.; Bronchalo-Vicente, L.; Freire, J.; Frande-Cabanes, E.; Alaez-Alvarez, L.; Gomez-Roman, J.; Yañez-Diaz, S.; Alvarez-Dominguez, C. Exceptional anti-neoplastic activity of a dendritic-cell targeted vaccine loaded with a Listeria peptide proposed against metastatic melanoma. Oncotarget 2015, 7, 16855–16865. [Google Scholar] [CrossRef] [Green Version]
- Terán-Navarro, H.; Calderon-Gonzalez, R.; Salcines-Cuevas, D.; García, I.; Marradi, M.; Freire, J.; Salmon, E.; Portillo-Gonzalez, M.; Frande-Cabanes, E.; García-Castaño, A.; et al. Preclinical development of Listeria-based nanovaccines as immunotherapies for solid tumors: Insights from melanoma. Oncoimmunology 2018, 8, e1541534. [Google Scholar]
- Naran, K.; Nundalall, T.; Chetty, S.; Barth, S. Principles of Immunotherapy: Implications for Treatment Strategies in Cancer and Infectious Diseases. Front. Microbiol. 2018, 9, 3158. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.-G.; Li, Y.-M. Emerging Adjuvants for Cancer Immunotherapy. Front. Chem. 2020, 8, 601. [Google Scholar] [CrossRef]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.I.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E.; et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017, 170, 1109–1119.e10. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.W.; Quail, D.F. Immunotherapy for Glioblastoma: Current Progress and Challenges. Front. Immunol. 2021, 12, 676301. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Li, Y.; Yang, J.; Jin, S.; Chen, G.; Li, D.; Fan, X.; Lin, H. Immunotherapy for Hepatocellular Carcinoma: Current Limits and Prospects. Front. Oncol. 2021, 11, 589680. [Google Scholar] [CrossRef]
- Alexandrov, L.; Nik-Zainal, S.; Wedge, D.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Klemm, F.; Maas, R.R.; Bowman, R.L.; Kornete, M.; Soukup, K.; Nassiri, S.; Brouland, J.-P.; Iacobuzio-Donahue, C.A.; Brennan, C.; Tabar, V.; et al. Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells. Cell 2020, 181, 1643–1660.e17. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Joseph, M.; Enting, D. Immune Responses in Bladder Cancer-Role of Immune Cell Populations, Prognostic Factors and Therapeutic Implications. Front. Oncol. 2019, 9, 1270. [Google Scholar] [CrossRef] [Green Version]
- De Liaño, G.A.; Duran, I. The continuing role of chemotherapy in the management of advanced urothelial cancer. Ther. Adv. Urol. 2018, 10, 455–480. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Ma, C.; Xu, P.; Guo, K.; Xu, A.; Liu, C. Efficacy of intravesical Bacillus Calmette-Guérin therapy against tumor immune escape in an orthotopic model of bladder cancer. Exp. Ther. Med. 2014, 9, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Noguera-Ortega, E.; Rabanal, R.M.; Gómez-Mora, E.; Cabrera, C.; Luquin, M.; Julián, E. Intravesical Mycobacterium brumae triggers both local and systemic immunotherapeutic responses against bladder cancer in mice. Sci. Rep. 2018, 8, 15102. [Google Scholar] [CrossRef]
- Wood, L.M.; Guirnalda, P.D.; Seavey, M.M.; Paterson, Y. Cancer immunotherapy using Listeria monocytogenes and listerial virulence factors. Immunol. Res. 2008, 42, 233–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacco, J.J.; Evans, M.; Harrington, K.J.; Man, S.; Powell, N.; Shaw, R.J.; Jones, T.M. Systemic listeriosis following vaccination with the attenuated Listeria monocytogenes therapeutic vaccine, ADXS11-001. Hum. Vaccines Immunother. 2015, 12, 1085–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Flores, K.G.; Vivanco-Cid, H. Biological Effects of Listeriolysin O: Implications for Vaccination. BioMed Res. Int. 2015, 2015, 360741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDougal, C.; Sauer, J.-D. Listeria monocytogenes: The Impact of Cell Death on Infection and Immunity. Pathogens 2018, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Maueröder, C.; Chaurio, R.A.; Dumych, T.; Podolska, M.; Lootsik, M.D.; Culemann, S.; Friedrich, R.P.; Bilyy, R.; Alexiou, C.; Schett, G.; et al. A blast without power-cell death induced by the tuberculosis-necrotizing toxin fails to elicit adequate immune responses. Cell Death Differ. 2016, 23, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ávila, O.; Hijazi, K.; Marradi, M.; Clavel, C.; Campion, C.; Kelly, C.; Penadés, S. Gold manno-glyconanoparticles: Multivalent systems to block HIV-1 gp120 binding to the lectin DC-SIGN. Chem. Eur. J. 2009, 15, 9874. [Google Scholar] [CrossRef]
- Rodriguez-Del Rio, E.; Marradi, M.; Calderon-Gonzalez, R.; Frande-Cabanes, E.; Penades, S.; Petrovsky, N.; Alvarez-Dominguez, C. A gold glyco-nanoparticle carrying a listeriolysin O peptide and formulated with AdvaxTM delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine 2015, 33, 1465. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Zeng, C.; Chen, Y.; Zhao, S.; Sfeir, M.Y.; Zhu, M.; Jin, R. Evolution from the plasmon to exciton state in ligand-protected atomically precise gold nanoparticles. Nat. Commun. 2016, 7, 13240. [Google Scholar] [CrossRef]
- Kuhn, S.; Hyde, E.J.; Yang, Y.; Rich, F.J.; Harper, J.L.; Kirman, J.R.; Ronchese, F. Increased numbers of monocyte-derived dendritic cells during successful tumour immunotherapy with immune-activating agents. J. Immunol. 2013, 191, 1984–1992. [Google Scholar] [CrossRef] [Green Version]
- Shinde, P.; Fernandes, S.; Melinkeri, S.; Kale, V.; Limaye, L. Compromised functionality of monocyte-derived dendritic cells in multiple myeloma patients may limit their use in cancer immunotherapy. Sci. Rep. 2018, 8, 5705. [Google Scholar] [CrossRef] [PubMed]
- Dmytryk, V.; Luhovska, T.; Yakovlev, P.; Savchuk, O.; Ostapchenko, L.; Halenova, T.; Raksha, N.; Tomchuk, V.; Vovk, T. Elevated levels of proinflammatory and anti-inflammatory cytokines in patients with bladder cancer depending on a tumor stage. J. Biol. Res. 2020, 93, 8632. [Google Scholar] [CrossRef]
- John, B.A.; Said, N. Insights from animal models of bladder cancer: Recent advances, challenges, and opportunities. Oncotarget 2017, 8, 57766–57781. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Patient Code | Clinical Symptoms and Treatment a | IL-12 b,* | IL-6 | IL-10 | TNF-α |
|---|---|---|---|---|---|
| MEL-1 | Metastatic melanoma (IV) NT | 0.3 ± 0.1 | 6.4 ± 0.9 | 5.6± 0.9 | 6.25 ± 0.1 |
| MEL-1 + GNP-LLO91–99 | Metastatic melanoma (IV) NT | 4.7 ± 0.1 | 2.22 ± 0.2 | 1.61 ± 0.1 | 66 ± 1.3 |
| MEL-2 | Nodular melanoma (IIIB) surgery | 0.5 ± 0.1 | 6.1 ± 0.1 | 6.6 ± 0.1 | 8.78 ± 0.1 |
| MEL-2 + GNP-LLO91–99 | Nodular melanoma (IIIB) surgery | 6.5 ± 0.2 | 2.07 ± 0.1 | 1.7 ± 0.1 | 50 ± 1.2 |
| BC-1 | Lung–bladder carcinoma Cisplatin–etoposide | 0.2 ± 0.1 | 6.0 ± 0.1 | 4.6 ± 0.1 | 7.8 ± 0.1 |
| BC-1 + GNP-LLO91–99 | Lung–bladder carcinoma Cisplatin–etoposide | 6.4 ± 0.1 | 2.3 ± 0.1 | 1.2 ± 0.1 | 220 ± 1.4 |
| BC-2 | Urothelial bladder carcinoma NT | 0.3 ± 0.2 | 7.1 ± 0.2 | 5.1 ± 0.2 | 8.1 ± 0.1 |
| BC-2 + GNP-LLO91–99 | Urothelial bladder carcinoma NT | 7.2 ± 0.3 | 2.1 ± 0.1 | 0.9 ± 0.2 | 204 ± 1.6 |
| CONT | NONE | 0.8 ± 0.1 | 3.1 ± 0.1 | 2.4 ± 0.1 | 2.0 ± 0.1 |
| CONT + GNP | NONE | 0.7 ± 0.1 | 2.8 ± 0.1 | 2.3 ± 0.1 | 1.8 ± 0.1 |
| CONT + GNP-LLO91–99 | NONE | 6.0 ± 0.2 | 4.1 ± 0.2 | 3.5 ± 0.1 | 150 ± 0.2 |
| Tumor Cell Line and Treatment a | Day 7 | Day 14 | Day 23 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SR c | U | TV d | SR | U | TV | SR | U | TV | |
| MB49 + GNP-LLO91–99 b | 100% ± 1 | - | 426 ± 5 | 100% ± 1 | - | 436 ± 11 | 100% ± 1 | - | 490 ± 10 |
| B16.F10 + GNP-LLO91–99 | 100% ± 1 | - | 460 ± 2 | 100% ± 2 | - | 470 ± 13 | 100% ± 2 | - | 490 ± 9 |
| MB49-NT | 85% ± 2 | - | 1080 ± 5 | 45% ± 5 | +/− | 1680 ± 16 | 16% ± 2 | + | 4921 ± 19 |
| B16.F10-NT | 84% ± 2 | - | 1090 ± 6 | 42% ± 2 | +/− | 1690 ± 19 | 10% ± 2 | + | 5322 ± 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terán-Navarro, H.; Zeoli, A.; Salines-Cuevas, D.; Marradi, M.; Montoya, N.; Gonzalez-Lopez, E.; Ocejo-Vinyals, J.G.; Dominguez-Esteban, M.; Gutierrez-Baños, J.L.; Campos-Juanatey, F.; et al. Gold Glyconanoparticles Combined with 91–99 Peptide of the Bacterial Toxin, Listeriolysin O, Are Efficient Immunotherapies in Experimental Bladder Tumors. Cancers 2022, 14, 2413. https://doi.org/10.3390/cancers14102413
Terán-Navarro H, Zeoli A, Salines-Cuevas D, Marradi M, Montoya N, Gonzalez-Lopez E, Ocejo-Vinyals JG, Dominguez-Esteban M, Gutierrez-Baños JL, Campos-Juanatey F, et al. Gold Glyconanoparticles Combined with 91–99 Peptide of the Bacterial Toxin, Listeriolysin O, Are Efficient Immunotherapies in Experimental Bladder Tumors. Cancers. 2022; 14(10):2413. https://doi.org/10.3390/cancers14102413
Chicago/Turabian StyleTerán-Navarro, Hector, Andrea Zeoli, David Salines-Cuevas, Marco Marradi, Noemi Montoya, Elena Gonzalez-Lopez, Javier Gonzalo Ocejo-Vinyals, Mario Dominguez-Esteban, Jose Luis Gutierrez-Baños, Felix Campos-Juanatey, and et al. 2022. "Gold Glyconanoparticles Combined with 91–99 Peptide of the Bacterial Toxin, Listeriolysin O, Are Efficient Immunotherapies in Experimental Bladder Tumors" Cancers 14, no. 10: 2413. https://doi.org/10.3390/cancers14102413
APA StyleTerán-Navarro, H., Zeoli, A., Salines-Cuevas, D., Marradi, M., Montoya, N., Gonzalez-Lopez, E., Ocejo-Vinyals, J. G., Dominguez-Esteban, M., Gutierrez-Baños, J. L., Campos-Juanatey, F., Yañez-Diaz, S., Garcia-Castaño, A., Rivera, F., Duran, I., & Alvarez-Dominguez, C. (2022). Gold Glyconanoparticles Combined with 91–99 Peptide of the Bacterial Toxin, Listeriolysin O, Are Efficient Immunotherapies in Experimental Bladder Tumors. Cancers, 14(10), 2413. https://doi.org/10.3390/cancers14102413