Liquid Biopsy in Head and Neck Cancer: Current Evidence and Future Perspective on Squamous Cell, Salivary Gland, Paranasal Sinus and Nasopharyngeal Cancers
Abstract
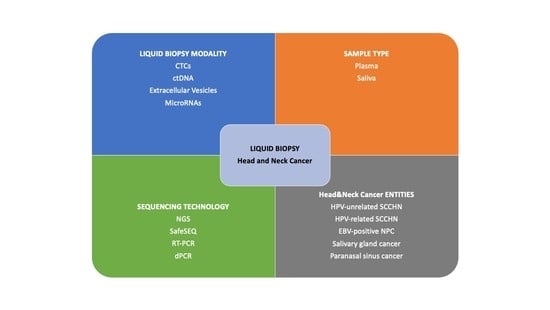
:Simple Summary
Abstract
1. Introduction
2. Current Evidence
2.1. Circulating Tumor Cells (CTC)
2.1.1. CTC in Squamous Cell Carcinoma of the Head and Neck
2.1.2. CTC in EBV+ Nasopharyngeal Cancer
2.1.3. CTC in Salivary Gland Cancer
2.1.4. CTC in Paranasal Sinus Cancer
2.2. Circulating Tumor DNA (ctDNA)
2.2.1. ctDNA in HPV-Unrelated Squamous Cell Carcinoma of the Head and Neck
2.2.2. ctDNA in HPV-Related Squamous Cell Carcinoma of the Head and Neck
2.2.3. ctDNA in EBV+ Nasopharyngeal Cancer (NPC)
2.3. Extracellular Vesicles
2.4. MicroRNAs
3. Future Perspective
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Remenar, E.; van Herpen, C.; Gorlia, T.; Mesia, R.; Degardin, M.; Stewart, J.S.; Jelic, S.; Betka, J.; Preiss, J.H.; et al. Cisplatin, Fluorouracil, and Docetaxel in Unresectable Head and Neck Cancer. N. Engl. J. Med. 2007, 357, 1695–1704. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Impact of nivolumab vs. standard, single-agent therapy of investigator’s choice on patient-reported outcomes in recurrent or metastatic squamous cell carcinoma of the head and neck: Health-related quality-of-life results from CheckMate 141, a randomized, phase 3 trial. Lancet Oncol. 2017, 18, 1104–1115. [Google Scholar] [CrossRef]
- Kong, L.; Birkeland, A.C. Liquid biopsies in head and neck cancer: Current state and future challenges. Cancers 2021, 13, 1874. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K.; Authors, C. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Wang, Y.; Springer, S.; Mulvey, C.L.; Silliman, N.; Schaefer, J.; Sausen, M.; James, N.; Rettig, E.M.; Guo, T.; Pickering, C.R.; et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci. Transl. Med. 2015, 7, 293ra104. [Google Scholar] [CrossRef] [Green Version]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.; Sougnez, C.; Mckenna, A.; et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, M.S.; Sougnez, C.; Lichtenstein, L.; Cibulskis, K.; Lander, E.; Gabriel, S.B.; Getz, G.; Ally, A.; Balasundaram, M.; Birol, I.; et al. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. Head and neck carcinoma immunotherapy: Facts and hopes. Clin. Cancer Res. 2018, 24, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Theodoraki, M.N.; Yerneni, S.; Gooding, W.E.; Ohr, J.; Clump, D.A.; Bauman, J.E.; Ferris, R.L.; Whiteside, T.L. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Oncoimmunology 2019, 8, e1593805. [Google Scholar] [CrossRef]
- Lin, J.C.; Tsai, C.S.; Wang, W.Y.; Jan, J.S. Detection of Circulating Tumor Cells in Venous Blood of Nasopharyngeal Carcinoma Patients by Nested Reverse Transcriptase-Polymerase Chain Reaction. Kaoshiung J. Med. Sci. 2000, 16, 1–8. [Google Scholar]
- Nakashima, H.; Yoshida, R.; Hirosue, A.; Kawahara, K.; Sakata, J.; Arita, H.; Yamamoto, T.; Toya, R.; Murakami, R.; Hiraki, A.; et al. Circulating miRNA-1290 as a potential biomarker for response to chemoradiotherapy and prognosis of patients with advanced oral squamous cell carcinoma: A single-center retrospective study. Tumor Biol. 2019, 41, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zong, J.; Lin, S.; Verhoeven, R.J.A.; Tong, S.; Chen, Y.; Ji, M.; Cheng, W.; Tsao, S.W.; Lung, M.; et al. Circulating Epstein-Barr virus microRNAs miR-BART7 and miR-BART13 as biomarkers for nasopharyngeal carcinoma diagnosis and treatment. Int. J. Cancer 2015, 136, E301–E312. [Google Scholar] [CrossRef]
- Ramayanti, O.; Verkuijlen, S.A.W.M.; Novianti, P.; Scheepbouwer, C.; Misovic, B.; Koppers-Lalic, D.; van Weering, J.; Beckers, L.; Adham, M.; Martorelli, D.; et al. Vesicle-bound EBV-BART13-3p miRNA in circulation distinguishes nasopharyngeal from other head and neck cancer and asymptomatic EBV-infections. Int. J. Cancer 2019, 144, 2555–2566. [Google Scholar] [CrossRef]
- Onidani, K.; Shoji, H.; Kakizaki, T.; Yoshimoto, S.; Okaya, S.; Miura, N.; Sekikawa, S.; Furuta, K.; Lim, C.T.; Shibahara, T.; et al. Monitoring of cancer patients via next-generation sequencing of patient-derived circulating tumor cells and tumor DNA. Cancer Sci. 2019, 110, 2590–2599. [Google Scholar] [CrossRef] [Green Version]
- Wirtschafter, A.; Benninger, M.S.; Moss, T.J.; Umiel, T.; Blazoff, K.; Worsham, M.J. Micrometastatic tumor detection in patients with head and neck cancer: A preliminary report. Arch. Otolaryngol.—Head Neck Surg. 2002, 128, 40–43. [Google Scholar] [CrossRef] [Green Version]
- Partridge, M.; Brakenhoff, R.; Phillips, E.; Ali, K.; Francis, R.; Hooper, R.; Lavery, K.; Brown, A.; Langdon, J. Detection of Rare Disseminated Tumor Cells Identifies Head and Neck Cancer Patients at Risk of Treatment Failure. Clin. Cancer Res. 2003, 9, 5287–5294. [Google Scholar]
- Guney, K.; Yoldas, B.; Ozbilim, G.; Derin, A.T.; Sarihan, S.; Balkan, E. Detection of micrometastatic tumor cells in head and neck squamous cell carcinoma. A possible predictor of recurrences? Saudi Med. J. 2007, 28, 216–220. [Google Scholar]
- Winter, S.C.; Stephenson, S.A.; Subramaniam, S.K.; Paleri, V.; Ha, K.; Marnane, C.; Krishnan, S.; Rees, G. Long term survival following the detection of circulating tumour cells in head and neck squamous cell carcinoma. BMC Cancer 2009, 9, 424. [Google Scholar] [CrossRef] [Green Version]
- Jatana, K.R.; Balasubramanian, P.; Lang, J.C.; Yang, L.; Jatana, C.A.; White, E.; Agrawal, A.; Ozer, E.; Schuller, D.E.; Teknos, T.N.; et al. Significance of circulating tumor cells in patients with squamous cell carcinoma of the head and neck: Initial results. Arch. Otolaryngol.—Head Neck Surg. 2010, 136, 1274–1279. [Google Scholar] [CrossRef] [Green Version]
- Buglione, M.; Grisanti, S.; Almici, C.; Mangoni, M.; Polli, C.; Consoli, F.; Verardi, R.; Costa, L.; Paiar, F.; Pasinetti, N.; et al. Circulating tumour cells in locally advanced head and neck cancer: Preliminary report about their possible role in predicting response to non-surgical treatment and survival. Eur. J. Cancer 2012, 48, 3019–3026. [Google Scholar] [CrossRef]
- Nichols, A.C.; Lowes, L.E.; Szeto, C.C.; Basmaji, J.; Dhaliwal, S.; Chapeskie, C.; Todorovic, B.; Read, N.; Venkatesan, V.; Hammond, A.; et al. Detection of circulating tumor cells in advanced head and neck cancer using the CellSearch system. Head Neck 2012, 34, 1440–1444. [Google Scholar] [CrossRef]
- Bozec, A.; Ilie, M.; Dassonville, O.; Long, E.; Poissonnet, G.; Santini, J.; Chamorey, E.; Ettaiche, M.; Chauvière, D.; Peyrade, F.; et al. Significance of circulating tumor cell detection using the CellSearch system in patients with locally advanced head and neck squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2745–2749. [Google Scholar] [CrossRef]
- He, S.; Li, P.; Long, T.; Zhang, N.; Yu, Z. Detection of circulating tumour cells with the CellSearch system in patients with advanced-stage head and neck cancer: Preliminary results. J. Laryngol. Otol. 2013, 127, 788–793. [Google Scholar] [CrossRef]
- Hsieh, J.C.; Lin, H.C.; Huang, C.Y.; Hsu, H.L.; Wu, T.M.; Lee, C.L.; Chen, M.C.; Wang, H.M.; Tseng, C. Prognostic value of circulating tumor cells with podoplanin expression in patients with locally advanced or metastatic head and neck squamous cell carcinoma. Head Neck 2015, 37, 1448–1455. [Google Scholar] [CrossRef]
- Hristozova, T.; Konschak, R.; Stromberger, C.; Fusi, A.; Liu, Z.; Weichert, W.; Stenzinger, A.; Budach, V.; Keilholz, U.; Tinhofer, I. The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN). Ann. Oncol. 2011, 22, 1878–1885. [Google Scholar] [CrossRef]
- Tinhofer, I.; Konschak, R.; Stromberger, C.; Raguse, J.D.; Dreyer, J.H.; Jöhrens, K.; Keilholz, U.; Budach, V. Detection of circulating tumor cells for prediction of recurrence after adjuvant chemoradiation in locally advanced squamous cell carcinoma of the head and neck. Ann. Oncol. 2014, 25, 2042–2047. [Google Scholar] [CrossRef]
- Grisanti, S.; Almici, C.; Consoli, F.; Buglione, M.; Verardi, R.; Bolzoni-Villaret, A.; Bianchetti, A.; Ciccarese, C.; Mangoni, M.; Ferrari, L.; et al. Circulating tumor cells in patients with recurrent or metastatic head and neck carcinoma: Prognostic and predictive significance. PLoS ONE 2014, 9, e103918. [Google Scholar] [CrossRef]
- Inhestern, J.; Oertel, K.; Stemmann, V.; Schmalenberg, H.; Dietz, A.; Rotter, N.; Veit, J.; Görner, M.; Sudhoff, H.; Junghanb, C.; et al. Prognostic role of circulating tumor cells during induction chemotherapy followed by curative surgery combined with postoperative radiotherapy in patients with locally advanced oral and oropharyngeal squamous cell cancer. PLoS ONE 2015, 10, e0132901. [Google Scholar] [CrossRef]
- Kusukawa, J.; Suefuji, Y.; Ryu, F.; Noguchi, R.; Iwamoto, O.; Kameyama, T. Dissemination of cancer cells into circulation occurs by incisional biopsy of oral squamous cell carcinoma. J. Oral Pathol. Med. 2000, 29, 303–307. [Google Scholar] [CrossRef]
- Garrel, R.; Mazel, M.; Perriard, F.; Vinches, M.; Cayrefourcq, L.; Guigay, J.; Digue, L.; Aubry, K.; Alfonsi, M.; Delord, J.P.; et al. Circulating Tumor Cells as a Prognostic Factor in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: The CIRCUTEC Prospective Study. Clin. Chem. 2019, 65, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Chikamatsu, K.; Tada, H.; Takahashi, H.; Kuwabara-Yokobori, Y.; Ishii, H.; Ida, S.; Shino, M. Expression of immune-regulatory molecules in circulating tumor cells derived from patients with head and neck squamous cell carcinoma. Oral Oncol. 2019, 89, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kulasinghe, A.; Perry, C.; Kenny, L.; Warkiani, M.E.; Nelson, C.; Punyadeera, C. PD-L1 expressing circulating tumour cells in head and neck cancers. BMC Cancer 2017, 17, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gröbe, A.; Blessmann, M.; Hanken, H.; Friedrich, R.E.; Schön, G.; Wikner, J.; Effenberger, K.E.; Kluwe, L.; Heiland, M.; Pantel, K.; et al. Prognostic relevance of circulating tumor cells in blood and disseminated tumor cells in bone marrow of patients with squamous cell carcinoma of the oral cavity. Clin. Cancer Res. 2014, 20, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strati, A.; Koutsodontis, G.; Papaxoinis, G.; Angelidis, I.; Zavridou, M.; Economopoulou, P.; Kotsantis, I.; Avgeris, M.; Mazel, M.; Perisanidis, C.; et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1923–1933. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Kenny, L.; Perry, C.; Thiery, J.P.; Jovanovic, L.; Vela, I.; Nelson, C.; Punyadeera, C. Impact of label-free technologies in head and neck cancer circulating tumour cells. Oncotarget 2016, 7, 71223–71234. [Google Scholar] [CrossRef] [Green Version]
- Dyavanagoudar, S.; Kale, A.; Bhat, K.; Hallikerimath, S. Reverse transcriptase polymerase chain reaction study to evaluate dissemination of cancer cells into circulation after incision biopsy in oral squamous cell carcinoma. Indian J. Dent. Res. 2008, 19, 315–319. [Google Scholar] [CrossRef]
- Liao, C.-J.; Hsieh, C.-H.; Hung, F.-C.; Wang, H.-M.; Chou, W.-P.; Wu, M.-H. The Integration of a Three-Dimensional Spheroid Cell Culture Operation in a Circulating Tumor Cell (CTC) Isolation and Purification Process: A Preliminary Study of the Clinical Significance and Prognostic Role of the CTCs Isolated from the Blood Samples of Head and Neck Cancer Patients. Cancers 2019, 11, 783. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Zhang, Y.; Guo, L.; Wang, S.; Fang, M.; Mao, W.; Lou, J. Evaluation of therapeutic efficacy with CytoSorter® circulating tumor cell-capture system in patients with locally advanced head and neck squamous cell carcinoma. Cancer Manag. Res. 2019, 11, 5857–5869. [Google Scholar] [CrossRef]
- Chang, P.H.; Wu, M.H.; Liu, S.Y.; Wang, H.M.; Huang, W.K.; Liao, C.T.; Yen, T.C.; Ng, S.H.; Chen, J.S.; Lin, Y.C.; et al. The prognostic roles of pretreatment circulating tumor cells, circulating cancer stem-like cells, and programmed cell death-1 expression on peripheral lymphocytes in patients with initially unresectable, recurrent or metastatic head and neck cancer: An Exploratory Study of Three Biomarkers in One-time Blood Drawing. Cancers 2019, 11, 540. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.M.; Wu, M.H.; Chang, P.H.; Lin, H.C.; Liao, C.D.; Wu, S.M.; Hung, T.M.; Lin, C.Y.; Chang, T.C.; Tzu-Tsen, Y..; et al. The change in circulating tumor cells before and during concurrent chemoradiotherapy is associated with survival in patients with locally advanced head and neck cancer. Head Neck 2019, 41, 2676–2687. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Takahashi, H.; Sakakura, K.; Ida, S.; Mito, I.; Toyoda, M.; Chikamatsu, K. Circulating tumor cells in patients with head and neck squamous cell carcinoma: Feasibility of detection and quantitation. Head Neck 2017, 39, 2180–2186. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.M.; Wang, X.; Qian, X.; Switchenko, J.M.; Nie, S.; Patel, K.R.; Cassidy, R.J.; Shin, D.M.; Beitler, J.J. Measurement of circulating tumor cells in squamous cell carcinoma of the head and neck and patient outcomes. Clin. Transl. Oncol. 2019, 21, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zou, K.; Yuan, Z.; Yang, C.; Lin, X.; Xiong, B. Clinicopathological and prognostic significance of circulating tumor cells in patients with esophageal cancer: A meta-analysis. OncoTargets Ther. 2017, 10, 3907–3916. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.P.; Bahig, H.; Wang, J.; Cardenas, C.E.; Lucci, A.; Hall, C.S.; Meas, S.; Sarli, V.N.; Yuan, Y.; Urbauer, D.L.; et al. Predicting treatment Response based on Dual assessment of magnetic resonance Imaging kinetics and Circulating Tumor cells in patients with Head and Neck cancer (PREDICT-HN): Matching “liquid biopsy” and quantitative tumor modeling. BMC Cancer 2018, 18, 903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Shi, H.; Jiang, T.; Liu, Z.; Lin, P.P.; Chen, N. Circulating tumor cells with karyotyping as a novel biomarker for diagnosis and treatment of nasopharyngeal carcinoma. BMC Cancer 2018, 18, 1133. [Google Scholar] [CrossRef]
- Si, Y.; Lan, G.; Deng, Z.; Wang, Y.; Lu, Y.; Qin, Y.; Huang, B.; Yang, Y.; Weng, J.; Han, X.; et al. Distribution and clinical significance of circulating tumor cells in nasopharyngeal carcinoma. Jpn. J. Clin. Oncol. 2016, 46, 622–630. [Google Scholar] [CrossRef] [Green Version]
- You, R.; Liu, Y.P.; Lin, M.; Huang, P.Y.; Tang, L.Q.; Zhang, Y.N.; Pan, Y.; Liu, W.L.; Guo, W.B.; Zou, X.; et al. Relationship of circulating tumor cells and Epstein–Barr virus DNA to progression-free survival and overall survival in metastatic nasopharyngeal carcinoma patients. Int. J. Cancer 2019, 145, 2873–2883. [Google Scholar] [CrossRef]
- Li, Y.J.; Luo, Y.; Xie, X.Q.; Li, P.; Wang, F. The prognostic value of COX-2 expression on circulating tumor cells in nasopharyngeal carcinoma: A prospective analysis. Radiother. Oncol. 2018, 129, 396–402. [Google Scholar] [CrossRef]
- Vo, J.H.; Nei, W.L.; Hu, M.; Phyo, W.M.; Wang, F.; Fong, K.W.; Tan, T.; Soong, Y.L.; Cheah, S.L.; Sommat, K.; et al. Comparison of Circulating Tumour Cells and Circulating Cell-Free Epstein-Barr Virus DNA in Patients with Nasopharyngeal Carcinoma Undergoing Radiotherapy. Sci. Rep. 2016, 6, 13. [Google Scholar] [CrossRef]
- He, C.; Huang, X.; Su, X.; Tang, T.; Zhang, X.; Ma, J.; Guo, X.; Lv, X. The association between circulating tumor cells and Epstein-Barr virus activation in patients with nasopharyngeal carcinoma. Cancer Biol. Ther. 2017, 18, 888–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Shen, C.; Wang, H.; Chen, F.; Li, G.; Wen, Z. Joint quantitative measurement of hTERT mRNA in both peripheral blood and circulating tumor cells of patients with nasopharyngeal carcinoma and its clinical significance. BMC Cancer 2017, 17, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.; Xing, S.; Li, J.; Zhang, L.; Chen, S. Circulating tumor cells: A valuable marker of poor prognosis for advanced nasopharyngeal carcinoma. Mol. Med. 2019, 25, 50. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Shi, J.; Zhu, W.; Wang, X. Association of Plasma Epstein-Barr Virus LMP1 and EBER1 with Circulating Tumor Cells and the Metastasis of Nasopharyngeal Carcinoma. Pathol. Oncol. Res. 2020, 26, 1893–1901. [Google Scholar] [CrossRef]
- Wen, Z.; Li, Z.; Yong, P.; Liang, D.; Xie, D.; Chen, H.; Yang, Y.; Wu, S.; Li, C.; Cheng, Z. Detection and clinical significance of circulating tumor cells in patients with nasopharyngeal carcinoma. Oncol. Lett. 2019, 18, 2537–2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Luo, Y.; Ma, X.L.; Li, S.; Liu, L.; Zhang, H.; Li, P.; Wang, F. Clinical significance of circulating tumor cells and their expression of cyclooxygenase-2 in patients with nasopharyngeal carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6951–6961. [Google Scholar] [CrossRef]
- Seethala, R.R.; Stenman, G. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumors: Tumors of the Salivary Gland. Head Neck Pathol. 2017, 11, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Song, S.; Lee, M.; Swatloski, T.; Kang, J.H.; Ko, Y.H.; Park, W.Y.; Jeong, H.S.; Park, K. Integrative genomic analysis of salivary duct carcinoma. Sci. Rep. 2020, 10, 14995. [Google Scholar] [CrossRef]
- Schmitt, N.C.; Kang, H.; Sharma, A. Salivary Duct Carcinoma: An aggressive Salivary Gland Malignancy with Opportunities for Targeted Therapy. Oral Oncol. 2017, 74, 40–48. [Google Scholar] [CrossRef]
- Asai, S.; Sumiyoshi, S.; Yamada, Y.; Tateya, I.; Nagao, T.; Minamiguchi, S.; Haga, H. High-grade salivary gland carcinoma with the ETV6-NTRK3 gene fusion: A case report and literature review of secretory carcinoma with high-grade transformation. Pathol. Int. 2021, 71, 427–434. [Google Scholar] [CrossRef]
- Fisher, B.M.; Tang, K.D.; Warkiani, M.E.; Punyadeera, C.; Batstone, M.D. A pilot study for presence of circulating tumour cells in adenoid cystic carcinoma. Int. J. Oral Maxillofac. Surg. 2021, 50, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, V.; Miodini, P.; Reduzzi, C.; Alfieri, S.; Daidone, M.G.; Licitra, L.; Locati, L.D. Tailoring treatment of salivary duct carcinoma (SDC) by liquid biopsy: ARv7 expression in circulating tumor cells. Ann. Oncol. 2018, 29, 1598–1600. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Camarero, S.; de la Orden García, V.; García-Barberán, V.; Mediero-Valeros, B.; Subhi-Issa, A.I.; Llovet García, P.; Bando-Polaino, I.; Merino-Menéndez, S.; Pérez-Segura, P.; Díaz-Rubio, E. Nasoethmoidal Intestinal-Type Adenocarcinoma Treated with Cetuximab: Role of Liquid Biopsy and BEAMing in Predicting Response to Anti-Epidermal Growth Factor Receptor Therapy. Oncologist 2019, 24, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.H.; Chang, K.W.; Kao, S.Y.; Cheng, H.W.; Liu, C.J. Increased plasma circulating cell-free DNA could be a potential marker for oral cancer. Int. J. Mol. Sci. 2018, 19, 3303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdomo, S.; Avogbe, P.H.; Foll, M.; Abedi-Ardekani, B.; Facciolla, V.L.; Anantharaman, D.; Chopard, P.; Le Calvez-Kelm, F.; Vilensky, M.; Polesel, J.; et al. Circulating tumor DNA detection in head and neck cancer: Evaluation of two different detection approaches. Oncotarget 2017, 8, 72621–72632. [Google Scholar] [CrossRef] [Green Version]
- Fostira, F.; Oikonomopoulou, P.; Kladi, A.; Edelstein, D.; Stieler, K.; Heim, D.; Gkotzamanidou, M.; Anastasiou, M.; Kotsantis, I.; Kavourakis, G.; et al. Blood-based testing of mutations in patients with head and neck squamous cell carcinoma (HNSCC) using highly sensitive SafeSeq technology. Ann. Oncol. 2019, 30 (Suppl. S5), v449–v474. [Google Scholar] [CrossRef]
- Flach, S.; Howarth, K.; Hackinger, S.; Pipinikas, C.; Ellis, P.; McLay, K.; Marsico, G.; Forshew, T.; Walz, C.; Reichel, C.A.; et al. Liquid BIOpsy for MiNimal RESidual DiSease Detection in Head and Neck Squamous Cell Carcinoma (LIONESS)—A personalised circulating tumour DNA analysis in head and neck squamous cell carcinoma. Br. J. Cancer 2022, 126, 1186–1195. [Google Scholar] [CrossRef]
- Li, W.; Wildsmith, S.; Ye, J.; Han, S.; Morsli, N.; He, P.; Shetty, J.; Yovine, A.J.; Holoweckyi, N.; Raja, R.; et al. Plasma-based tumor mutational burden (bTMB) as predictor for survival in phase III EAGLE study: Durvalumab (D) ± tremelimumab (T) versus chemotherapy (CT) in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) after platinum failure. ASCO Virtual Meeting 2020. J. Clin. Oncol. 2020, 38 (Suppl. S15), 6511. [Google Scholar] [CrossRef]
- Porter, A.; Natsuhara, M.; Daniels, G.A.; Patel, S.P.; Sacco, A.G.; Bykowski, J.; Banks, K.C.; Cohen, E.E. Next generation sequencing of cell free circulating tumor DNA in blood samples of recurrent and metastatic head and neck cancer patients. Transl. Cancer Res. 2020, 9, 203–209. [Google Scholar] [CrossRef]
- Wilson, H.L.; D’Agostino, R.B., Jr.; Meegalla, N.; Petro, R.; Commander, S.; Topaloglu, U.; Zhang, W.; Porosnicu, M. The prognostic and therapeutic value of the mutational profile of blood and tumor tissue in head and neck squamous cell carcinoma. Oncologist 2021, 26, e279–e289. [Google Scholar] [CrossRef]
- Van Ginkel, J.H.; Huibers, M.M.H.; van Es, R.J.J.; de Bree, R.; Willems, S.M. Droplet digital PCR for detection and quantification of circulating tumor DNA in plasma of head and neck cancer patients. BMC Cancer 2017, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Egyud, M.; Sridhar, P.; Devaiah, A.; Yamada, E.; Saunders, S.; Ståhlberg, A.; Filges, S.; Krzyzanowski, P.M.; Kalatskaya, I.; Jiao, W.; et al. Plasma circulating tumor DNA as a potential tool for disease monitoring in head and neck cancer. Head Neck 2019, 41, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Pall, A.H.; Jakobsen, K.K.; Gronhoj, C.; von Buchwald, C. Circulating tumour DNA alterations as biomarkers for head and neck cancer: A systematic review. Acta Oncol. 2020, 59, 845–850. [Google Scholar] [CrossRef]
- Hudecková, M.; Kouchy, V.; Rottenberg, J.; Gál, B. Gene Mutations in Circulating Tumour DNA as a Diagnostic and Prognostic Marker in Head and Neck Cancer—A Systematic Review. Biomedicines 2021, 9, 1548. [Google Scholar] [CrossRef] [PubMed]
- Damerla, R.R.; Lee, N.Y.; You, D.; Soni, R.; Shah, R.; Reyngold, M.; Katabi, N.; Wu, V.; McBride, S.M.; Tsai, C.J.; et al. Detection of Early Human Papillomavirus–Associated Cancers by Liquid Biopsy. JCO Precis. Oncol. 2019, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chera, B.S.; Kumar, S.; Beaty, B.T.; Marron, D.; Jefferys, S.; Green, R.; Goldman, E.C.; Amdur, R.; Sheets, N.; Dagan, R.; et al. Rapid Clearance Profile of Plasma Circulating Tumor HPV Type 16 DNA During Chemoradiotherapy Correlates With Disease Control in HPV-Associated Oropharyngeal Cancer. Clin. Cancer Res. 2019, 25, 4682–4690. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrom, K.R.; Li, G.; Hussey, C.S.; Vo, J.T.; Wei, Q.; Zhao, C.; Sturgis, E.M. Circulating human papillomavirus DNA as a marker for disease extent and recurrence among patients with oropharyngeal cancer. Cancer 2015, 121, 3455–3464. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Banh, A.; Kwok, S.; Shi, X.; Wu, S.; Krakow, T.; Khong, B.; Bavan, B.; Bala, P.; Pinsky, B.; et al. Quantitation of human papillomavirus DNA in plasma of oropharyngeal carcinoma patients. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e351–e358. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.M.; Chan, J.Y.K.; Zhang, Z.; Wang, H.; Khan, Z.; Bishop, J.A.; Westra, W.; Koch, W.M.; Califano, J.A. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol.—Head Neck Surg. 2014, 140, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Mazurek, A.M.; Rutkowski, T.; Fiszer-Kierzkowska, A.; Małusecka, E.; Składowski, K. Assessment of the total cfDNA and HPV16/18 detection in plasma samples of head and neck squamous cell carcinoma patients. Oral Oncol. 2016, 54, 36–41. [Google Scholar] [CrossRef]
- Siravegna, G.; O’Boyle, C.J.; Varmeh, S.; Queenan, N.; Michel, A.; Stein, J.; Thierauf, J.; Sadow, P.M.; Faquin, W.C.; Perry, S.K.; et al. Cell-Free HPV DNA Provides an Accurate and Rapid Diagnosis of HPV-Associated Head and Neck Cancer. Clin. Cancer Res. 2022, 28, 719–727. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, C.J.; Siravegna, G.; Varmeh, S.; Queenan, N.; Michel, A.; Chang Sing Pang, K.; Stein, J.; Thierafu, J.C.; Sadow, P.M.; Faquin, W.C.; et al. Cell-free papillomavirus DNA kinetics after surgery for human papillomavirus-associated oropharyngeal cancer. Cancer 2022, 128, 2193–2204. [Google Scholar] [CrossRef]
- Akashi, K.; Sakai, T.; Fukuoka, O.; Saito, Y.; Yoshida, M.; Ando, M.; Ito, T.; Murakami, Y.; Yamasoba, T. Usefulness of circulating tumor DNA by targeting human papilloma virus-derived sequences as a biomarker in p16-positive oropharyngeal cancer. Sci. Rep. 2022, 12, 572. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-Y.; Guo, S.-Y.; Tang, L.-Q.; Lu, T.-Y.; Chen, B.-L.; Zhong, Q.-Y.; Zou, M.-S.; Tang, Q.-N.; Chen, W.-H.; Guo, S.S.; et al. Combination of Tumor Volume and Epstein-Barr Virus DNA Improved Prognostic Stratification of Stage II Nasopharyngeal Carcinoma in the Intensity Modulated Radiotherapy Era: A Large-Scale Cohort Study. Cancer Res. Treat. 2018, 50, 861–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Tang, L.Q.; Chen, Q.-Y.; Lu, H.; Guo, S.-S.; Liu, L.-T.; Guo, L.; Mo, H.-Y.; Zhao, C.; Guo, X.; et al. Plasma Epstein-Barr Viral DNA Complements TNM Classification of Nasopharyngeal Carcinoma in the Era of Intensity-Modulated Radiotherapy. Oncotarget 2016, 7, 6221–6230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, R.; Tang, L.L.; Mao, Y.P.; Du, X.J.; Chen, L.; Zhang, Z.C.; Liu, L.-Z.; Tian, L.; Luo, X.-T.; Xie, Y.-B.; et al. Proposed modifications and incorporation of plasma Epstein-Barr virus DNA improve the TNM staging system for Epstein-Barr virus-related nasopharyngeal carcinoma. Cancer 2019, 125, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Lee, V.H.F.; Kwong, D.L.W.; Leung, T.W.; Choi, C.W.; O’Sullivan, B.; Lam, K.O.; Lai, V.; Khong, P.-L.; Chan, S.-K.; Ng, C.-Y.; et al. The addition of pretreatment plasma Epstein–Barr virus DNA into the eighth edition of nasopharyngeal cancer TNM stage classification. Int. J. Cancer 2019, 144, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, S.; Li, W.F.; Chen, L.; Mao, Y.P.; Guo, Y.; Liu, Q.; Ma, J.; Tang, L.-L. Selection and Validation of Induction Chemotherapy Beneficiaries Among Patients With T3N0, T3N1, T4N0 Nasopharyngeal Carcinoma Using Epstein-Barr Virus DNA: A Joint Analysis of Real-World and Clinical Trial Data. Front. Oncol. 2019, 9, 1343. [Google Scholar] [CrossRef]
- Huang, C.L.; Sun, Z.Q.; Guo, R.; Liu, X.; Mao, Y.P.; Peng, H.; Tian, L.; Lin, A.-H.; Li, L.; Shao, J.-Y.; et al. Plasma Epstein-Barr Virus DNA Load After Induction Chemotherapy Predicts Outcome in Locoregionally Advanced Nasopharyngeal Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 355–361. [Google Scholar] [CrossRef]
- Liu, L.T.; Tang, L.Q.; Chen, Q.Y.; Lu, Z.; Guo, S.S.; Guo, L.; Mo, H.-Y.; Zhao, C.; Guo, X.; Cao, K.-J.; et al. The prognostic value of plasma Epstein-Barr viral DNA and tumor response to neoadjuvant chemotherapy in advanced-stage nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Peng, H.; Li, W.F.; Zhang, Y.; Liu, L.Z.; Tian, L.; Lin, A.-H.; Sun, Y.; Ma, J. Individualized induction chemotherapy by pre-treatment plasma Epstein-Barr viral DNA in advanced nasopharyngeal carcinoma. BMC Cancer 2018, 18, 1276. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.C.A.; Woo, J.K.S.; King, A.; Zee, B.C.Y.; Lam, W.K.J.; Chan, S.L.; Chu, S.W.I.; Mak, C.; Tse, I.O.L.; Leung, S.Y.M.; et al. Analysis of plasma Epstein–Barr virus DNA to screen for nasopharyngeal cancer. N. Engl. J. Med. 2017, 377, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Camarero, S.; Pérez-Alfayate, R.; Puebla, F.; Cabrera-Martín, M.N.; Pérez-Segura, P. Increased clinical and plasma EBV DNA responses to platinum-gemcitabine after nivolumab in patients with heavily platinum-pretreated nasopharyngeal cancer. Oral Oncol. 2020, 103, 104527. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical significance of PD-L1 þ exosomes in plasma of head and neck cancer patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef] [Green Version]
- Theodoraki, M.N.; Laban, S.; Jackson, E.K.; Lotfi, R.; Schuler, P.J.; Brunner, C.; Hoffmann, T.K.; Whiteside, T.L.; Hofmann, L. Changes in circulating exosome molecular profiles following surgery/(chemo)radiotherapy: Early detection of response in head and neck cancer patients. Br. J. Cancer 2021, 125, 1677–1686. [Google Scholar] [CrossRef]
- Theodoraki, M.N.; Yerneni, S.S.; Brunner, C.; Theodorakis, J.; Hoffmann, T.K.; Whiteside, T.L. Plasma-derived exosomes reverse epithelial-to-mesenchimal transition after photodynamic therapy of patients with head and neck cancer. Oncoscience 2018, 5, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhou, Y.; Lu, J.; Sun, Y.; Xiao, H.; Liu, M.; Tian, L. Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med. Oncol. 2014, 31, 148. [Google Scholar] [CrossRef]
- Kang, J.W.; Eun, Y.G.; Lee, Y.C. Diagnostic value of salivary miRNA in head and neck squamous cell cancer: Systematic review and meta-analysis. Int. J. Mol. Sci. 2021, 22, 7026. [Google Scholar] [CrossRef]
- Rapado-González, O.; Martínez-Reglero, C.; Salgado-Barreira, A.; López-López, R.; Suárez-Cunqueiro, M.M.; Muinelo-Romay, L. miRNAs in liquid biopsy for oral squamous cell carcinoma diagnosis: Systematic review and meta-analysis. Oral Oncol. 2019, 99, 104465. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chang, J.T.C.; Huang, Y.C.; Huang, C.C.; Chen, W.H.; Lee, L.Y.; Huang, B.-S.; Chen, Y.-J.; Li, H.-F.; Cheng, A.-J. Combined determination of circulating miR-196a and miR-196b levels produces high sensitivity and specificity for early detection of oral cancer. Clin. Biochem. 2015, 48, 115–121. [Google Scholar] [CrossRef]
- Liu, C.J.; Lin, S.C.; Yang, C.C.; Cheng, H.W.; Chang, K.W. Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck 2012, 34, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Fayda, M.; Isin, M.; Tambas, M.; Guveli, M.; Meral, R.; Altun, M.; Sahin, D.; Ozkan, G.; Sanli, Y.; Isin, H.; et al. Do circulating long non-coding RNAs (lncRNAs)(LincRNA-p21, GAS 5, HOTAIR) predict the treatment response in patients with head and neck cnacer treated with chemoradiotherapy? Tumor Biol. 2016, 37, 3969–3978. [Google Scholar] [CrossRef] [PubMed]
- Lubov, J.; Maschietto, M.; Ibrahim, I.; Mlynarek, A.; Hier, M.; Kowalski, L.P.; Alaoui-Jamali, M.A.; da Silva, S.D. Meta-analysis of microRNAs expression in head and neck cancer: Uncovering association with outcome and mechanisms. Oncotarget 2017, 8, 55511–55524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lousada-Fernandez, F.; Rapado-Gonzalez, O.; Lopez-Cedrun, J.L.; Lopez-Lopez, R.; Muinelo-Romay, L.; Suarez-Cunqueiro, M.M. Liquid biopsy in oral cancer. Int. J. Mol. Sci. 2018, 19, 1704. [Google Scholar] [CrossRef] [Green Version]
- Adeola, H.A.; Bello, I.O.; Aruleba, R.T.; Francisco, N.M.; Adekiya, T.A.; Adefuye, A.O.; Ikwegbue, P.C.; Musaigwa, F. The Practicality of the Use of Liquid Biopsy in Early Diagnosis and Treatment Monitoring of Oral Cancer in Resource-Limited Settings. Cancers 2022, 14, 1139. [Google Scholar] [CrossRef]
| Author | CTC Detection Technology | N | Stage | N (Samples) | Detection Rate | Prognostic Value |
|---|---|---|---|---|---|---|
| Wirtshafter (2002) [16] | CellSearch | 18 | I-IV | 18 | 44% (0–3 CTC) | - |
| Partridge (2003) [17] | IA with negative enrichment | 36 | I-IV | 36 | 50% (0–5 CTC) | Worse DFS |
| Guney (2007) [18] | CellSearch | 21 | LA | 21 | 33% | - |
| Winter (2009) [19] | ISET (size) | 16 | LA | 32 (pre- and post-SX) | 63% | - |
| Jatana (2010) [20] | IA with negative enrichment | 48 | I-IV | 61 | 71% | Worse DFS |
| Buglione (2012) [21] | CellSearch | 73 | I-IV | 41 (pre- y post-TX) | 15% (0–43 CTC) | Decrease in CTC → better respone |
| Nichols (2012) [22] | CellSearch | 15 | LA | 15 | 40% | Worse OS in CTC+ |
| Bozec (2013) [23] | CellSearch | 49 (LA) 10 (HC) | LA | 49 LA SCCHN 10 HC | 16% SCCHN 0% in N(-) 23% in N1-2c 0% HC | - |
| He (2013) [24] | CellSearch | 9 | III-I | 9 | 33% (0–1 CTC) | - |
| Hsieh (2015) [25] | IA with negative enrichment | 53 | LA or R/M | 53 | 19% | - |
| Hristozova (2011) [26] | Fluid Cytometry | 42 | Unresectable LA | 42 | 43% | Association of CTC+ with N+ |
| Gröbe (2013) [34] | CellSearch | 110 | Resected (R0) OSCC | 110 | - | Association of CTC+ with N+ and <PFS |
| Tinhofer (2014) [27] | Immunoaffinity through tumor-antigen amplification | 144 | Resected | 144 | 29% | Association of CTC+ with <DFS and OS |
| Grisanti (2014) [28] | CellSearch | 53 | R/M | 53 | 26% | Association of CTC+ with <DFS and OS |
| Inhesten (2015) [29] | Fluid Cytometry | 40 | II-IV | 120 (before, during and after TX) | 97% (80% at baseline) | Association of CTC+ with <DFS and OS |
| Dyavanagoudar (2008) [37] | Detection of CK19 with RT-PCR | 25 | LA OSCC | 25 | 16% | - |
| Kusukawa (2000) [30] | Detection of CK19 with RT-PCR | 20 | LA OSCC | 20 | 10% | - |
| Garrel (2019) [31] | CellSearch, EPISPOT, Fluid Cytometry | 65 | R/M | Baseline, d7 and d21 after first cycle of EXTREME | EPISPOT: 69%, CellSearch: 21% CMF: 11% | Association of stability/increase of CTC with EPISPOT or CTC+ with CellSearch with <SLP |
| Strati (2017) [35] | CellSearch | 113 | LA | Baseline, after iCT and after CRT | PDL1 overexpression in CTC in 25.5% (baseline), 23.5% (after iCT) and 22.2% (after CRT) | Associaton of PDL1+ CTC post-CRT with <PFS and OS |
| Chikamatsu (2019) [32] | IA with negative enrichment and mRNA expression of epithelial markers (CK19, EpCAM, EGFR, c-MET) | 30 | R/M | 30 | CTC with epithelial marker expression ≥ 1:80% | - |
| Kulasinghe (2017) [33] | Spiral microfluidic system | 1 | R/M | 1 | Detection of CTC “clusters” with PDL1 expression | - |
| Kulasinghe (2016) [36] | IA vs. NIA: CellSearch (IA) vs. ScreenCell (NIA) vs. RosetteSep (NIA) | 43 | R/M | 43 | CellSearch: 18.6% (8/43) ScreenCell: 46.4% (13/28) RosetteSep 64% (16/25) | - |
| Liao (2019) [38] | IA with negative enrichment | 20 | LA or R/M | 20 | Detection of CTC with epithelial phenotype (E-CTC) in 75% and CTC with mesenchymal phenotype (M-CTC) in 90% | Association of M-CTC with higher odds of distant metastases and shorter PFS |
| Zheng (2019) [39] | CytoSorter (IA + microfluidic system) | 20 (LA) 6 (LTB) 12 (HC) | LA | Pre- and post-iCT | HC: 0% CTC+ SCCHN: 77% CTC+ | Association of CTC with N+, PR vs. CR and <DFS |
| Chang (2019) [40] | IA with negative enrichment | 34 | R/M | 34 | - | <DFS and OS and a higher CTC count |
| Wang (2019) [41] | IA with negative enrichment | 53 | LA | Before and during CRT | - | Association of CTC reduction with longer PFS and OS |
| Kawada (2017) [42] | CellSieve (Low pressure microfiltration system) | 32 | R/M | 32 | 90.6% | Association of higher number of CTC with more advanced stage |
| Onidani (2019) [15] | LFIMA (microfluidic and inertial detection system) vs. ctDNA | 9 | R/M | 9 | Mutations in CTC in 4/9 pts and in ctDNA in 1/9 pts | - |
| Morgan (2019) [43] | SERS (RAMAN-type spectroscopy) | 82 | LA | 82 | - | Association of higher number of CTC with DMFS |
| Sun (2017) [44] | Meta-analysis of 17 CTC studies in SCCHN | - | - | - | - | Association of higher number of CTC with <PFS and <OS |
| Author | CTC Detection Technology | N (Patients) | Stage | N (Samples) | Detection Rate | Prognostic Value |
|---|---|---|---|---|---|---|
| Zhang (2018) [46] | SE-iFISH | 50 | II-IV | Pre- and post-CT | 92% | Higher CTC count with a higher TNM/AJCC stage CTC count correlated with tumor response Cr 8 aneuploidy in CTC associated with CT efficacy |
| Zhang (2018) [46] | ISET | 33 | I-IV | 33 | 66.7% | Good correlation of CTC count with EBV ctDNA |
| Si (2016) [47] | CanPatrol™ (size detection method) EBV-ctDNA detected with RT-PCR | 81 | I-IV | 81 | CTC detection: 96.6% 3 phenotypes: hybrid: >expression of EBV proteins, mesenquimal: >MMP-9 expression | Higher CTC count correlated with N+ and M1 disease |
| You (2019) [48] | CTC vs. EBV-ctDNA | 148 R/M 122 LA | 148 R/M 122 LA | Pre- and post-CT | M0: 19/122 (16%) M1: 64/148 (43.2%) | CTC and EBV-ctDNA have prognostic value that increases when combined between them and with tumor imaging tests |
| Li (2018) [49] | CTC and COX-2 expression in CTC | 131 | LA | Pre- and post-CRT | 66.4% COX2+ CTC at baseline 46.1% COX2+ CTC post-CRT | < COX-2 expression post-CRT Worse prognosis with COX2+ CTC post-CRT |
| Vo (2016) [50] | CTC (Microsieve) vs. EBV-ctDNA (qPCR and dPCR) | 46 | LA | Pre- and post-CRT | EBV-ctDNA: qPCR BamHi-W 89%, qPCR EBNA1 69%, dPCR EBNA1 85% | Better correlation of clinical stage, radiological response and OS with EBV-ctDNA compared to CTC count |
| He (2017) [51] | ISET + IHQ CK5/CK6/P36 ISET + ISH EBERs | 33 | LA | Baseline | CTC: 66.7% CTM: 6.1% | Correlation of CTC count and titles of EBV VCA-IgA and EBV-ctDNA |
| Fu (2017) [52] | mRNA-hTERT in plasma and in CTC | 33 NPC 24 HC | LA | Pre- and post-CRT | - | Association of mRNA-hTERT in plasma and CTC with clinical stage and response to therapy |
| Wen (2019) [55] | CanPatrol technology | 60 NPC 18 HC | LA | Pre- and post-iCT | CTC+: 86.7% CTCmesenq+: 50% | Reduction of CTC count with iCT Worse prognosis with CTCmesenq+ |
| Sun (2019) [54] | CellSpoter Analyzer vs. EBV-ctDNA (RT-PCR) | M0: 114 M1: 136 | M0 & M1 | Baseline | Median number of CTC: M0: 9.3 M1: 12.5 | CTC count and LMP1, BART and EBER1 levels higher in M1 vs. M0 |
| Ou (2019) [53] | CellSearch | 370 | III-IV | Baseline | M0: 77/288 (27%) M1: 16/81 (20%) | Worse prognosis with higher CTC count Combination of CTC and EBV-ctDNA have a better prognostic value |
| Xie (2019) [56] | CanPatrolTM + HIS (COX-2) vs. EBV-ctDNA (RT-qPCR) | 50 II-IV 10 HC | II-IV | 50 NPC 10 HC | CTC+: 96% M-CTC+: 76% | High CTC, M-CTC+ and COX2-CTC+ more frequent in stage IV and with EBV-ctDNA |
| Author | N | Setting | Technology | Detection Rate | Specificity | Conclusions |
|---|---|---|---|---|---|---|
| Lin (2018) [64] | 121 SCCHN (OC) 50 HC | LA | dPCR | - | - | ctDNA level higher in SCCHN vs. HC Higher levels of ctDNA with higher T stage and N stage Reduction in levels of ctDNA in 75% of cases after surgical resection |
| Van Ginkel (2017) [71] | 6 SCCHN | LA | dPCR | 100% | 100% | Detection of mutations in 100% of cases |
| Egyud (2019) [72] | 8 SCCHN | LA | NGS | 6/8 | - | Relapse in 4/8 pts, in 2 of them ctDNA detectable before relapse |
| Perdomo (2017) [65] | 36 SCCHN plasma | LA | NGS (TP53, NOTCH1, CDKN2A, CASP8, PTEN) | 18/36 (67%) (stages I and II) | - | Detection of 18 concordant mutations between primary tumor and plasma |
| Perdomo (2017) [65] | 37 SCCHN plama & oral rinses | LA | NGS (TP53) | 3/37 | - | Low concordance of TP53 mutations between the primary tumor, plasma, and oral washings |
| Fostira (2019) [66] | 54 LA SCCHN 15 R/M SCCHN | LA and R/M | SAFESEQ (TP53, CDKN2A, PIK3CA, HRAS) | LA: 51% R/M: 93% | - |
|
| Flach (2022) [67] | 17 LA SCCHN | LA | FFPE: WES Plasma: NGS deep sequencing (RaDaRTM) | Pre-SX: 100% Post-SX: | - | High tumor-plasma concordance in pre-SX samples All relapses showed post-SX ctDNA positive samples |
| Li (2020) [68] | 247 R/M SCCHN | R/M (2nd line) | NGS Pre-TX | - | - | High bTMB (≥16 muts/Mb) predicted a longer OS and PFS with IO. Mutations in KMT2D or ATM in plasma ctDNA predicted longer OS with Durvalumab + Tremelimumab compared to CT |
| Porter (2020) [69] | 60 R/M HNC (21 OPC, 8 SGC, 4 Thyroid cancer) | R/M | NGS (Guardant360) | SCCHN: 66% SGC: 50% Thyroid: 100% | - | Most common mutations in plasma: TP53 (68%), PIK3CA (34%), NOTCH1 (20%), ARID1A (15%). These results were concondant with tumor NGS, although 73% had blood alterations not identified in tissue. Alterations found in ctDNA allowed to inform management decisions. |
| Wilson (2021) [70] | 75 R/M SCCHN | R/M | NGS | 65% with actionable ctDNA alterations | - | Concordance among altered genes between tumor and ctDNA was 13% ctDNA alterations were significantly associated with decreased OS and presence and extent of disease at last visit. |
| Author (Year) | N | Setting | Technology | Detection Rate | Specificity | Conclusions |
|---|---|---|---|---|---|---|
| Damerla (2019) [75] | 97 HPV(+) SCCHN (OPC) 6 HPV(−) SCCHN 20 HC | Localized | dPCR | 95.6% | 100% | ctDNA detected HPV16 and 33 with same accuracy that in tissue HPV-ctDNA also detectable in small tumors |
| Chera (2019) [76] | 103 SCCHN | Localized | dPCR | 89% | 97% | None of the pts with ≥ 95% of ctDNA clearance relapsed 35% of the pts with <95% of ctDNA clearance relapsed HPV-ctDNA should be explored as a marker for deintesification strategies in HPV(+) disease |
| Mazurek (2016) [80] | 200 SCCHN (HPV(+) and HPV(−)) | Localized | RT-PCR (TERT amplification and HPV16/HPV18) | 14% HPV+ in plasma | - | Higher HPV-ctDNA levels in OPC vs. other locations Higher HPV-ctDNA levels with higher stages Similar ctDNA levels in HPV(+) and HPV(−) SCCHN |
| Wang (2015) [6] | 93 SCCHN | Localized | HPV(+): PCR digital (E6 y E7), PCR multiplex (E6 y E7) HPV(−): NGS (TP53,PIK3CA, CDKN2A,FBXW7, HRAS, NRAS) | L/LA: 10/10 (100%) R/M: 37/39 (95%) Saliva: OC (100%), Other (47–70%) Plasma: OC (80%), Other (86%) | - | High detection rate in plasma and saliva ctDNA detection in 3/3 cases that relapsed and in 0/5 cases that did not relapse |
| Dahlstrom (2015) [77] | 262 SCCHN | Localized (I-IV) | RT-PCR | 60.5% | 67% | Baseline HPV-ctDNA associated with global and N stage Baseline HPV-ctDNA was not a predictor of relapse |
| Cao (2012) [78] | 40 HPV(+) 24 HPV(−) 10 HC | Localized | RT-PCR | 65% | - | HPV-ctDNA negativization after RT in 14 pts Increase in HPV-ctDNA in 3 pts with metastatic relapse |
| Ahn (2014) [79] | 93 plasma and saliva pre- and post-TX (81 HPV(+) y 12 HPV(−)) | Localized | RT-PCR | 67% | 89% | OS shorter in pts with detectable HPV-ctDNA post-TX in plasma or saliva |
| Siravegna (2021) [81] | 61 HPV(+) SCCHN 45 HPV(−) SCCHN 25 HC | LA newly diagnosed SCCHN | ddPCR (HPV 16,18,33,35, 45) | 98.4% | 98.6% | Very high detection rates, with lower cost and earlier diagnosis compared to standard clinical workup |
| O’Boyle (2022) [82] | 33 | L/LA treated with surgery | ddPCR (HPV 16, 18, 33, 35, 45) | - | - | ctDNAHPV levels on POD 1 were associated with residual disease |
| Akashi (2022) [83] | 25 HPV(+) | L/LA newly diagnosed SCCHN | dPCR (E6 & E7 regions of HPV DNA) | 56% | - | 56% detection rate at baseline. 0% detection rate after treatment. In 2 relapsing patients, HPV-specific ctDNA was positive. |
| Author | N | Setting | Technology | Detection Rate | Conclusions |
|---|---|---|---|---|---|
| Chen (2018) [84] | 385 | Stage II NPC | RT-qPCR | 161/385 (41.8%) | EBV-ctDNA levels and tumor volume allows to classify stage II NPC into favorable and unfavorable prognostic groups |
| Zhang (2015) [85] | 1467 | Stage I-IVB NPC | RT-qPCR | - | EBV-ctDNA levels complement TNM improving its prognostic value |
| Guo (2019) [86] | 1529 | Stage I-IVA NPC | RT-qPCR | - | EBV-ctDNA levels complement TNM improving its prognostic value |
| Lee (2019) [87] | 518 | Stage I-IVA NPC | RT-PCR | Median baseline EBV-ctDNA: 588 copies/mL | EBV-ctDNA levels complement TNM 8ª Ed improving its prognostic value |
| Liu (2015) [90] | 185 | Stage III-IVA NPC | RT-qPCR | Pre- iCT: 89% Post-iCT: 31% | Detectable EBV-ctDNA post-iCT associate with a worse Px |
| Xu (2019) [88] | 2692 | Stage III-IVA NPC | RT-qPCR | Pre- iCT EBV-ctDNA ≥ 2000 copies/mL: 57.5% | High levels of EBV-ctDNA pre-iCT associate with a worse Px and identify a group that benefits from iCT |
| Huang (2019) [89] | 278 | Stage III-IV NPC | RT-qPCR | Pre-iCT median EBV-ctDNA levels: 9035 copies/mL Post-iCT: 23.7% | High levels of EBV-ctDNA post-iCT associate with a worse Px |
| Zhang (2018) [91] | 4482 | Stage III-IVB NPC | RT-qPCR | Median EBV-ctDNA: 3740 copies/mL | High levels of EBV-ctDNA before iCT identified a poor-prognosis group that benefits from iCT |
| Chan (2017) [92] | 20,349 screened → 309 ctDNA EBV+ | Stage I-IVB NPC | RT-qPCR | Screening: 309/(5.5%) | Screening in an endemic population allowed to augment the % of detected cases in early stage (I–II) and this associated with a better survival. |
| Cabezas-Camarero (2020) [93] | 2 | Stage IV NPC | RT-qPCR | 100% | Levels of EBV-ctDNA associated with response to CT post-IO |
| ClinicalTrials.gov (Accessed on 15 May 2022) (Other Study IDs) | Design | N | Sample Type | Primary Endpoint | Secondary Endpoint | Enrollment Status |
|---|---|---|---|---|---|---|
| NCT05122507 (KOHACIN study) | Prospective cohort study | 200 | Blood and saliva | Early recurrence detection lead time (time between liquid biopsy-based recurrence detection and clinical recurrence or progression) | RFS, OS | Recruiting |
| NCT03942380 | Prospective cohort study | 500 | Blood (ctDNA, RNA, HPV-ctDNA) | % of HNC (all histologies) detected using liquid biopsy in blood % of HNC (all histologies) recurrence detected using liquid biopsy in blood | - | Recruiting |
| NCT03702309 (LIBERATE study) | Prospective cohort study | 2500 (Several cancer types including HNC) | Archived tissue and peripheral blood | Collection and annotation of biospecimens at the Princess Margaret Cancer Center | Implement an electronic informed consent process for clinical research and correlative studies questionnaire at the Princess Margaret Cancer Center | Active, not recruiting |
| NCT04606940 (IO-KIN) | Prospective cohort study | 20 (SCCHN) | Archived tissue and peripheral blood | Evaluate the kinetics of ctDNA in advanced/metastatic. SCCHN treated with anti-PD1 agents |
| Recruiting |
| NCT04490564 (CBS-PD-L1a) | Prospective cohort study | 25 | Archived tissue and peripheral blood | Clinical performance of PD-L1 kit in CTCs of peripheral blood and tumor tissue samples. | Correlations between PD-L1 expression in serial liquid samples with patients’ responsiveness to therapy. | Recruiting |
| NCT05059444 (ORACLE) | Prospective cohort study | 1000 (Several cancer types including HNC) | Archived tissue and peripheral blood | DRFi is defined as the time from the end of primary treatment until the time of diagnosis of a distant recurrence of the Index Cancer. | Sensitivity, PPV, Lead time defined as the interval between ctDNA detection and clinical detection of recurrence. | Recruiting |
| NCT04599309 (PRE-MERIDAN) | Prospective cohort study | 20 (LA SCCHN candidates for standard definitive therapy) | Archived tissue and serially-collected peripheral blood | Number of high-risk LA-HNSCC patients with successful detection of ctDNA and/or HPV DNA in real time |
| Recruiting |
| NCT03712566 (MASST-001) | Prospective cohort study | 39 (Several cancer types including HNC) | Archived tissue and peripheral blood | To comprehensively characterize genomic, epigenetic and immune profiling features and changes in longitudinal blood samples that are associated with systemic treatment of recurrent or metastatic SCCHN. |
| Active, not recruiting |
| NCT05150509 | Prospective cohort study | 110 OSCC | Saliva | To establish a diagnostic test in the early detection of OSCC | - | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabezas-Camarero, S.; Pérez-Segura, P. Liquid Biopsy in Head and Neck Cancer: Current Evidence and Future Perspective on Squamous Cell, Salivary Gland, Paranasal Sinus and Nasopharyngeal Cancers. Cancers 2022, 14, 2858. https://doi.org/10.3390/cancers14122858
Cabezas-Camarero S, Pérez-Segura P. Liquid Biopsy in Head and Neck Cancer: Current Evidence and Future Perspective on Squamous Cell, Salivary Gland, Paranasal Sinus and Nasopharyngeal Cancers. Cancers. 2022; 14(12):2858. https://doi.org/10.3390/cancers14122858
Chicago/Turabian StyleCabezas-Camarero, Santiago, and Pedro Pérez-Segura. 2022. "Liquid Biopsy in Head and Neck Cancer: Current Evidence and Future Perspective on Squamous Cell, Salivary Gland, Paranasal Sinus and Nasopharyngeal Cancers" Cancers 14, no. 12: 2858. https://doi.org/10.3390/cancers14122858
APA StyleCabezas-Camarero, S., & Pérez-Segura, P. (2022). Liquid Biopsy in Head and Neck Cancer: Current Evidence and Future Perspective on Squamous Cell, Salivary Gland, Paranasal Sinus and Nasopharyngeal Cancers. Cancers, 14(12), 2858. https://doi.org/10.3390/cancers14122858