Artificial Intelligence Predictive Models of Response to Cytotoxic Chemotherapy Alone or Combined to Targeted Therapy for Metastatic Colorectal Cancer Patients: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
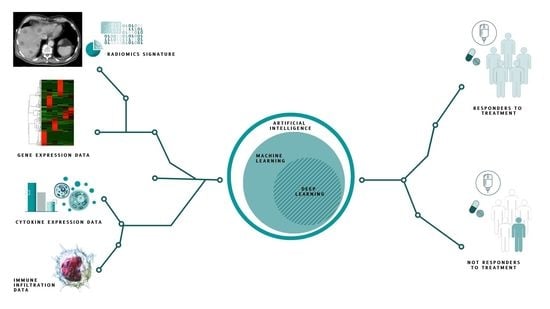
1.1. Machine and Deep Learning Techniques
1.2. Standard Chemotherapy, Targeted Therapy and Mutational Profiles
2. Methods
2.1. Search Strategy and Data Extraction
2.2. Study Selection
2.3. Objectives
2.4. Publication Quality
2.5. Rational Framework
2.6. Statistical Analysis
3. Results
3.1. Outcomes and Performance Estimates of Predictive Models
3.2. Publication Validation
3.3. Artificial Intelligence Predictive Models
3.4. Cytotoxic Chemotherapy and Radiomics Learning Models
3.5. Cytotoxic Chemotherapy Alone and Biomarkers and Clinical Learning Models
3.6. Targeted Therapy and Radiomics Learning Models
3.7. Targeted Therapy and Biomarkers Learning Models
3.8. Meta-Analysis
3.9. Predictive Model Performances
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Hitre, E.; Zaluski, J.; Chien, C.-R.C.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef]
- Mullard, A. Stemming the tide of drug resistance in cancer. Nat. Rev. Drug Discov. 2020, 19, 221–223. [Google Scholar] [CrossRef]
- Bruera, G.; Ricevuto, E. Toxicity Syndromes, Patient-Related Clinical Indicator of Toxicity Burden Induced by Intensive Triplet Chemotherapy-Based Regimens in Gastrointestinal Cancers with Metastatic Disease. Front. Oncol. 2020, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Kopetz, S.; Kuboki, Y.; Kim, T.W.; Munster, P.N.; Krauss, J.C.; Falchook, G.S.; Han, S.-W.; Heinemann, V.; Muro, K.; et al. Sotorasib for previously treated colorectal cancers with KRASG12C mutation (CodeBreaK100): A prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2021, 23, 115–124. [Google Scholar] [CrossRef]
- Raghav, K.; Loree, J.M.; Morris, J.S.; Overman, M.J.; Yu, R.; Meric-Bernstam, F.; Menter, D.; Korphaisarn, K.; Kee, B.; Muranyi, A.; et al. Validation of HER2 Amplification as a Predictive Biomarker for Anti–Epidermal Growth Factor Receptor Antibody Therapy in Metastatic Colorectal Cancer. JCO Precis. Oncol. 2019, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Okamura, R.; Boichard, A.; Kato, S.; Sicklick, J.K.; Bazhenova, L.; Kurzrock, R. Analysis of NTRK Alterations in Pan-Cancer Adult and Pediatric Malignancies: Implications for NTRK-Targeted Therapeutics. JCO Precis. Oncol. 2018, 2, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Haibe, Y.; Kreidieh, M.; El Hajj, H.; Khalifeh, I.; Mukherji, D.; Temraz, S.; Shamseddine, A. Resistance Mechanisms to Anti-angiogenic Therapies in Cancer. Front. Oncol. 2020, 10, 221. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Javed, Z.; Sadia, H.; Qureshi, I.A.; Irshad, A.; Ahmed, R.; Malik, K.; Raza, S.; Abbas, A.; Pezzani, R.; et al. Clinical applications of artificial intelligence and machine learning in cancer diagnosis: Looking into the future. Cancer Cell Int. 2021, 21, 270. [Google Scholar] [CrossRef]
- Wiemken, T.L.; Kelley, R.R. Machine Learning in Epidemiology and Health Outcomes Research. Annu. Rev. Public Health 2020, 41, 21–36. [Google Scholar] [CrossRef]
- Kourou, K.; Exarchos, K.P.; Papaloukas, C.; Sakaloglou, P.; Exarchos, T.; Fotiadis, D.I. Applied machine learning in cancer research: A systematic review for patient diagnosis, classification and prognosis. Comput. Struct. Biotechnol. J. 2021, 19, 5546–5555. [Google Scholar] [CrossRef]
- Munnia, A.; Giese, R.W.; Polvani, S.; Galli, A.; Cellai, F.; Peluso, M.E. Bulky DNA Adducts, Tobacco Smoking, Genetic Susceptibility, and Lung Cancer Risk. Adv. Clin. Chem. 2017, 81, 231–277. [Google Scholar]
- Ceppi, M.; Munnia, A.; Cellai, F.; Bruzzone, M.; Peluso, M.E. Linking the generation of DNA adducts to lung cancer. Toxicology 2017, 390, 160–166. [Google Scholar] [CrossRef]
- Brancato, B.; Munnia, A.; Cellai, F.; Ceni, E.; Mello, T.; Bianchi, S.; Catarzi, S.; Risso, G.G.; Galli, A.; Peluso, M.E.M. 8-Oxo-7,8-dihydro-2′-deoxyguanosine and other lesions along the coding strand of the exon 5 of the tumour suppressor gene P53 in a breast cancer case-control study. DNA Res. 2016, 23, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Munnia, A.; Cellai, F.; Tarocchi, M.; Ceni, E.; van Schooten, F.J.; Godschalk, R.; Giese, R.W.; Peluso, M. Ligation-Mediated Polymerase Chain Reaction Detection of 8-Oxo-7,8-Dihydro-2′-Deoxyguanosine and 5-Hydroxycytosine at the Codon 176 of the p53 Gene of Hepatitis C-Associated Hepatocellular Carcinoma Patients. Int. J. Mol. Sci. 2020, 21, 6753. [Google Scholar] [CrossRef] [PubMed]
- Carozzi, F.M.; Bisanzi, S.; Carrozzi, L.; Falaschi, F.; Pegna, A.L.; Mascalchi, M.; Picozzi, G.; Peluso, M.; Sani, C.; Greco, L.; et al. Multimodal lung cancer screening using the ITALUNG biomarker panel and low dose computed tomography. Results of the ITALUNG biomarker study. Int. J. Cancer 2017, 141, 94–101. [Google Scholar] [CrossRef]
- Agudo, A.; Peluso, M.; Munnia, A.; Luján-Barroso, L.; Sánchez, M.-J.; Molina-Montes, E.; Sánchez-Cantalejo, E.; Navarro, C.; Tormo, M.-J.; Chirlaque, M.-D.; et al. Aromatic DNA Adducts and Risk of Gastrointestinal Cancers: A Case–Cohort Study within the EPIC–Spain. Cancer Epidemiol. Biomark. Prev. 2012, 21, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Badic, B.; Tixier, F.; le Rest, C.C.; Hatt, M.; Visvikis, D. Radiogenomics in Colorectal Cancer. Cancers 2021, 13, 973. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, M.R.; Gillies, R.J. The Biological Meaning of Radiomic Features. Radiology 2021, 298, 505–516. [Google Scholar] [CrossRef]
- Sarker, I.H. Machine Learning: Algorithms, Real-World Applications and Research Directions. SN Comput. Sci. 2021, 2, 160. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. Model inference and averaging. In The Elements of Statistical Learning: Data Mining, Inference, Prediction Springer Series in Statistics; Springer: New York, NY, USA, 2009; pp. 261–294. [Google Scholar] [CrossRef]
- Lynch, C.; Liston, C. New machine-learning technologies for computer-aided diagnosis. Nat. Med. 2018, 24, 1304–1305. [Google Scholar] [CrossRef]
- Parimbelli, E.; Marini, S.; Sacchi, L.; Bellazzi, R. Patient similarity for precision medicine: A systematic review. J. Biomed. Informat. 2018, 83, 87–96. [Google Scholar] [CrossRef]
- Tran, K.A.; Kondrashova, O.; Bradley, A.; Williams, E.D.; Pearson, J.V.; Waddell, N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021, 13, 152. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2019, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B.; Stintzing, S.; Marshall, J.; Yoshino, T.; de Gramont, A. Metastatic colorectal cancer: Advances in the folate-fluoropyrimidine chemotherapy backbone. Cancer Treat. Rev. 2021, 98, 102218. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.-S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2–negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, W.; Yue, L.; Dai, X.; Rong, D.; Wu, F.; Gu, J.; Qian, X. Perspectives on Immunotherapy of Metastatic Colorectal Cancer. Front. Oncol. 2021, 11, 659964. [Google Scholar] [CrossRef]
- Amado, R.G.; Wolf, M.; Peeters, M.; van Cutsem, E.; Siena, S.; Freeman, D.J.; Juan, T.; Sikorski, R.; Suggs, S.; Radinsky, R.; et al. Wild-Type KRAS Is Required for Panitumumab Efficacy in Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2008, 26, 1626–1634. [Google Scholar] [CrossRef]
- Bylsma, L.C.; Gillezeau, C.; Garawin, T.A.; Kelsh, M.A.; Fryzek, J.P.; Sangaré, L.; Lowe, K.A. Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: A systematic review and meta-analysis. Cancer Med. 2019, 9, 1044–1057. [Google Scholar] [CrossRef]
- De Roock, W.; Claes, B.; Bernasconi, D.; de Schutter, J.; Biesmans, B.; Fountzilas, G.; Kalogeras, K.T.; Kotoula, V.; Papamichael, D.; Laurent-Puig, P.; et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010, 11, 753–762. [Google Scholar] [CrossRef]
- De Roock, W.; de Vriendt, V.; Normanno, N.; Ciardiello, F.; Tejpar, S. KRAS, BRAF, PIK3CA, and PTEN mutations: Implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2010, 12, 594–603. [Google Scholar] [CrossRef]
- Tran, N.H.; Cavalcante, L.L.; Lubner, S.J.; Mulkerin, D.L.; LoConte, N.K.; Clipson, L.; Matkowskyj, K.A.; Deming, D.A. Precision medicine in colorectal cancer: The molecular profile alters treatment strategies. Ther. Adv. Med. Oncol. 2015, 7, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef]
- Morelli, C.; Formica, V.; Riondino, S.; Russo, A.; Ferroni, P.; Guadagni, F.; Roselli, M. Irinotecan or Oxaliplatin: Which is the First Move for the Mate? Curr. Med. Chem. 2021, 28, 3158–3172. [Google Scholar] [CrossRef]
- Dienstmann, R.; Vermeulen, L.; Guinney, J.; Kopetz, S.; Tejpar, S.; Tabernero, J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 2017, 17, 79–92, Erratum in Nat. Rev. Cancer 2017, 17, 268. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability–High/Mismatch Repair–Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair–Deficient/Microsatellite Instability–High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- A Diaz, L.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Andre, T.; Amonkar, M.; Norquist, J.M.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; A Punt, C.J.; Smith, D.; Garcia-Carbonero, R.; et al. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 665–677. [Google Scholar] [CrossRef]
- Lenz, H.-J.; van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.K.; Parseghian, C.M.; Escano, M.; Johnson, B.; Raghav, K.P.S.; Dasari, A.; Huey, R.; Overman, M.J.; Willis, J.; Lee, M.S.; et al. Phase I/II trial of encorafenib, cetuximab, and nivolumab in patients with microsatellite stable, BRAFV600E metastatic colorectal cancer. J. Clin. Oncol. 2022, 40, 12. [Google Scholar] [CrossRef]
- Tabernero, J.; Grothey, A.; van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E–Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef]
- Grassadonia, A.; di Marino, P.; Ficorella, C.; Cortellini, A.; Cannita, K.; Parisi, A.; Gamucci, T.; Zoratto, F.; Vici, P.; Barba, M.; et al. Impact of primary tumor location in patients with RAS wild-type metastatic colon cancer treated with first-line chemotherapy plus anti-EGFR or anti-VEGF monoclonal antibodies: A retrospective multicenter study. J. Cancer 2019, 10, 5926–5934. [Google Scholar] [CrossRef]
- Tejpar, S.; Stintzing, S.; Ciardiello, F.; Tabernero, J.; van Cutsem, E.; Beier, F.; Esser, R.; Lenz, H.-J.; Heinemann, V. Prognostic and Predictive Relevance of Primary Tumor Location in Patients with RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2017, 3, 194–201. [Google Scholar] [CrossRef]
- Bahl, A.; Talwar, V.; Sirohi, B.; Mehta, P.; Arya, D.; Shrivastava, G.; Dahiya, A.; Pavithran, K. Primary Tumor Location as a Prognostic and Predictive Marker in Metastatic Colorectal Cancer (mCRC). Front. Oncol. 2020, 10, 964. [Google Scholar] [CrossRef]
- Baran, B.; Ozupek, N.M.; Tetik, N.Y.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef]
- Johnston, P.G.; Fisher, E.R.; Rockette, H.E.; Fisher, B.; Wolmark, N.; Drake, J.C.; Chabner, B.A.; Allegra, C.J. The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. J. Clin. Oncol. 1994, 12, 2640–2647. [Google Scholar] [CrossRef]
- Salonga, D.; Danenberg, K.D.; Johnson, M.; Metzger, R.; Groshen, S.; Tsao-Wei, D.D.; Lenz, H.-J.; Leichman, C.G.; Leichman, L.; Diasio, R.B.; et al. Colorectal Tumors Responding to 5-Fluorouracil Have Low Gene Expression Levels of Dihydropyrimidine Dehydrogenase, Thymidylate Synthase, and Thymidine Phosphorylase1. Clin. Cancer Res. 2000, 6, 1322–1327. [Google Scholar]
- Popat, S.; Matakidou, A.; Houlston, R.S. Thymidylate Synthase Expression and Prognosis in Colorectal Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2004, 22, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Morganti, M.; Ciantelli, M.; Giglioni, B.; Putignano, A.L.; Nobili, S.; Papi, L.; Landini, I.; Napoli, C.; Valanzano, R.; Cianchi, F.; et al. Relationships between promoter polymorphisms in the thymidylate synthase gene and mRNA levels in colorectal cancers. Eur. J. Cancer 2005, 41, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, M.; Nobili, S.; Napoli, C.; Putignano, A.L.; Morganti, M.; Papi, L.; Valanzano, R.; Cianchi, F.; Tonelli, F.; Mazzei, T.; et al. Thymidylate synthase expression and genotype have no major impact on the clinical outcome of colorectal cancer patients treated with 5-fluorouracil. Pharmacol. Res. 2011, 64, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Nobili, S.; Napoli, C.; Landini, I.; Morganti, M.; Cianchi, F.; Valanzano, R.; Tonelli, F.; Cortesini, C.; Mazzei, T.; Mini, E. Identification of potential pharmacogenomic markers of clinical efficacy of 5-fluorouracil in colorectal cancer. Int. J. Cancer 2010, 128, 1935–1945. [Google Scholar] [CrossRef]
- Mini, E.; Lapucci, A.; Perrone, G.; D’Aurizio, R.; Napoli, C.; Brugia, M.; Landini, I.; Tassi, R.; Picariello, L.; Simi, L.; et al. RNA sequencing reveals PNN and KCNQ1OT1 as predictive biomarkers of clinical outcome in stage III colorectal cancer patients treated with adjuvant chemotherapy. Int. J. Cancer 2019, 145, 2580–2593. [Google Scholar] [CrossRef]
- Lapucci, A.; Perrone, G.; di Paolo, A.; Napoli, C.; Landini, I.; Roviello, G.; Calosi, L.; Naccarato, A.G.; Falcone, A.; Bani, D.; et al. PNN and KCNQ1OT1 Can Predict the Efficacy of Adjuvant Fluoropyrimidine-Based Chemotherapy in Colorectal Cancer Patients. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2020, 28, 631–644. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Isella, C.; Brundu, F.G.; Bellomo, S.E.; Galimi, F.; Zanella, E.R.; Porporato, R.; Petti, C.; Fiori, A.; Orzan, F.N.; Senetta, R.; et al. Selective analysis of cancer-cell intrinsic transcriptional traits defines novel clinically relevant subtypes of colorectal cancer. Nat. Commun. 2017, 8, 15107. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Luo, W.; Phung, Q.-D.; Tran, T.; Gupta, S.; Rana, S.; Karmakar, C.; Shilton, A.; Yearwood, J.L.; Dimitrova, N.; Ho, T.B.; et al. Guidelines for Developing and Reporting Machine Learning Predictive Models in Biomedical Research: A Multidisciplinary View. J. Med. Internet Res. 2016, 18, e323. [Google Scholar] [CrossRef]
- Brnabic, A.; Hess, L.M. Systematic literature review of machine learning methods used in the analysis of real-world data for patient-provider decision making. BMC Med. Informat. Decis. Mak. 2021, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Giannini, V.; Rosati, S.; DeFeudis, A.; Balestra, G.; Vassallo, L.; Cappello, G.; Mazzetti, S.; de Mattia, C.; Rizzetto, F.; Torresin, A.; et al. Radiomics predicts response of individual HER2 -amplified colorectal cancer liver metastases in patients treated with HER2 -targeted therapy. Int. J. Cancer 2020, 147, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Giannini, V.; Pusceddu, L.; Defeudis, A.; Nicoletti, G.; Cappello, G.; Mazzetti, S.; Sartore-Bianchi, A.; Siena, S.; Vanzulli, A.; Rizzetto, F.; et al. Delta-Radiomics Predicts Response to First-Line Oxaliplatin-Based Chemotherapy in Colorectal Cancer Patients with Liver Metastases. Cancers 2022, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.E.; Munnia, A.; Ceppi, M. Bisphenol-A exposures and behavioural aberrations: Median and linear spline and meta-regression analyses of 12 toxicity studies in rodents. Toxicology 2014, 325, 200–208. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Peluso, M.; Ceppi, M.; Munnia, A.; Puntoni, R.; Parodi, S. Analysis of 13 (32)P-DNA postlabeling studies on occupational cohorts exposed to air pollution. Am. J. Epidemiol. 2001, 153, 546–558. [Google Scholar] [CrossRef]
- Nakanishi, R.; Oki, E.; Hasuda, H.; Sano, E.; Miyashita, Y.; Sakai, A.; Koga, N.; Kuriyama, N.; Nonaka, K.; Fujimoto, Y.; et al. ASO Author Reflection: Radiomics-Based Prediction for the Responder to First-Line Oxaliplatin-Based Chemotherapy in Patients with Colorectal Liver Metastasis. Ann. Surg. Oncol. 2021, 28, 2986–2987. [Google Scholar] [CrossRef]
- Wei, J.; Cheng, J.; Gu, D.; Chai, F.; Hong, N.; Wang, Y.; Tian, J. Deep learning-based radiomics predicts response to chemotherapy in colorectal liver metastases. Med. Phys. 2020, 48, 513–522. [Google Scholar] [CrossRef]
- Defeudis, A.; Cefaloni, L.; Giannetto, G.; Cappello, G.; Rizzetto, F.; Panic, J.; Barra, D.; Nicoletti, G.; Mazzetti, S.; Vanzulli, A.; et al. Comparison of radiomics approaches to predict resistance to 1st line chemotherapy in liver metastatic colorectal cancer. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 3305–3308. [Google Scholar]
- Tsuji, S.; Midorikawa, Y.; Takahashi, T.; Yagi, K.; Takayama, T.; Yoshida, K.; Sugiyama, Y.; Aburatani, H. Potential responders to FOLFOX therapy for colorectal cancer by Random Forests analysis. Br. J. Cancer 2011, 106, 126–132. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; He, W.-Z.; Peng, L.-X.; Jia, W.-H.; Guo, R.-P.; Xia, L.-P.; Qian, C.-N. A prognostic classifier consisting of 17 circulating cytokines is a novel predictor of overall survival for metastatic colorectal cancer patients. Int. J. Cancer 2014, 136, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shen, H.; Li, M.-D.; Tan, C.-W.; Yu, J.-K.; Fang, X.-F.; Zheng, S. Identification of the biomarkers for the prediction of efficacy in the first-line chemotherapy of metastatic colorectal cancer patients using SELDI-TOF-MS and artificial neural networks. J. Clin. Oncol. 2012, 30, e14026. [Google Scholar] [CrossRef]
- Lu, L.; Dercle, L.; Zhao, B.; Schwartz, L.H. Deep learning for the prediction of early on-treatment response in metastatic colorectal cancer from serial medical imaging. Nat. Commun. 2021, 12, 6654. [Google Scholar] [CrossRef]
- Maaref, A.; Romero, F.P.; Montagnon, E.; Cerny, M.; Nguyen, B.; Vandenbroucke, F.; Soucy, G.; Turcotte, S.; Tang, A.; Kadoury, S. Predicting the Response to FOLFOX-Based Chemotherapy Regimen from Untreated Liver Metastases on Baseline CT: A Deep Neural Network Approach. J. Digit. Imaging 2020, 33, 937–945. [Google Scholar] [CrossRef]
- Dercle, L.; Lu, L.; Schwartz, L.H.; Qian, M.; Tejpar, S.; Eggleton, P.; Zhao, B.; Piessevaux, H. Radiomics Response Signature for Identification of Metastatic Colorectal Cancer Sensitive to Therapies Targeting EGFR Pathway. J. Natl. Cancer Inst. 2020, 112, 902–912. [Google Scholar] [CrossRef]
- Vera-Yunca, D.; Girard, P.; Guillen, Z.P.P.; Munafo, A.; Trocóniz, I.F.; Terranova, N. Machine Learning Analysis of Individual Tumor Lesions in Four Metastatic Colorectal Cancer Clinical Studies: Linking Tumor Heterogeneity to Overall Survival. AAPS J. 2020, 22, 58. [Google Scholar] [CrossRef]
- Barat, A.; Smeets, D.; Moran, B.; Zhang, W.; Cao, S.; Das, S.; Klinger, R.; Betge, J.; Murphy, V.; Bacon, O.; et al. Combination of variations in inflammation- and endoplasmic reticulum-associated genes as putative biomarker for bevacizumab response in KRAS wild-type colorectal cancer. Sci. Rep. 2020, 10, 9778. [Google Scholar] [CrossRef]
- Ubels, J.; Schaefers, T.; Punt, C.; Guchelaar, H.-J.; de Ridder, J. RAINFOREST: A random forest approach to predict treatment benefit in data from (failed) clinical drug trials. Bioinformatics 2020, 36, i601–i609. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; de Stefano, A.; Ottaiano, A.; Sbordone, C.; Brunese, L.; Izzo, F.; Petrillo, A. Radiomics-Derived Data by Contrast Enhanced Magnetic Resonance in RAS Mutations Detection in Colorectal Liver Metastases. Cancers 2021, 13, 453. [Google Scholar] [CrossRef]
- Del Rio, M.; Molina, F.; Bascoul-Mollevi, C.; Copois, V.; Bibeau, F.; Chalbos, P.; Bareil, C.; Kramar, A.; Salvetat, N.; Fraslon, C.; et al. Gene Expression Signature in Advanced Colorectal Cancer Patients Select Drugs and Response for the Use of Leucovorin, Fluorouracil, and Irinotecan. J. Clin. Oncol. 2007, 25, 773–780. [Google Scholar] [CrossRef]
- Oyaga-Iriarte, E.; Insausti, A.; Sayar, O.; Aldaz, A. Prediction of irinotecan toxicity in metastatic colorectal cancer patients based on machine learning models with pharmacokinetic parameters. J. Pharmacol. Sci. 2019, 140, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, L.; Weng, S.; Guo, C.; Dang, Q.; Xu, H.; Wang, L.; Lu, T.; Zhang, Y.; Sun, Z.; et al. Machine learning-based integration develops an immune-derived lncRNA signature for improving outcomes in colorectal cancer. Nat. Commun. 2022, 13, 816. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, F.; Lu, S.; Chen, G. Identification of Two Subgroups of FOLFOX Resistance Patterns and Prediction of FOLFOX Response in Colorectal Cancer Patients. Cancer Investig. 2020, 39, 62–72. [Google Scholar] [CrossRef]
- Li, C.; Chen, L.; Chou, C.; Ngorsuraches, S.; Qian, J. Using Machine Learning Approaches to Predict Short-Term Risk of Cardiotoxicity Among Patients with Colorectal Cancer After Starting Fluoropyrimidine-Based Chemotherapy. Cardiovasc. Toxicol. 2021, 22, 130–140. [Google Scholar] [CrossRef]
- Williams, C.; Seligmann, J.F.; Guetter, C.; Zhang, L.; Yan, D.; Muranyi, A.; Bai, I.; Singh, S.; Elliott, F.; Shires, M.; et al. Artificial intelligence-assisted immunohistochemical (IHC) evaluation of tumor amphiregulin (AREG) and epiregulin (EREG) expression as a combined predictive biomarker for panitumumab (Pan) therapy benefit in RAS wild-type (wt) metastatic colorectal cancer (mCRC): Analysis within the phase III PICCOLO trial. J. Clin. Oncol. 2021, 39, 111. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, D.; Ye, M.; Sun, L.; Zhang, X.; Mm, X.L.; Nie, P.; Xing, B.; Sun, Y. Deep learning-assisted magnetic resonance imaging prediction of tumor response to chemotherapy in patients with colorectal liver metastases. Int. J. Cancer 2020, 148, 1717–1730. [Google Scholar] [CrossRef]
- Abraham, J.P.; Magee, D.; Cremolini, C.; Antoniotti, C.; Halbert, D.D.; Xiu, J.; Stafford, P.; Berry, D.A.; Oberley, M.J.; Shields, A.F.; et al. Clinical Validation of a Machine-learning–derived Signature Predictive of Outcomes from First-line Oxaliplatin-based Chemotherapy in Advanced Colorectal Cancer. Clin. Cancer Res. 2021, 27, 1174–1183. [Google Scholar] [CrossRef]
- Johnson, H.; El-Schich, Z.; Ali, A.; Zhang, X.; Simoulis, A.; Wingren, A.G.; Persson, J.L. Gene-Mutation-Based Algorithm for Prediction of Treatment Response in Colorectal Cancer Patients. Cancers 2022, 14, 2045. [Google Scholar] [CrossRef]
- Lu, W.; Fu, D.; Kong, X.; Huang, Z.; Hwang, M.; Zhu, Y.; Chen, L.; Jiang, K.; Li, X.; Wu, Y.; et al. FOLFOX treatment response prediction in metastatic or recurrent colorectal cancer patients via machine learning algorithms. Cancer Med. 2020, 9, 1419–1429. [Google Scholar] [CrossRef]
- Naseem, M.; Cao, S.; Yang, D.; Millstein, J.; Puccini, A.; Loupakis, F.; Stintzing, S.; Cremolini, C.; Tokunaga, R.; Battaglin, F.; et al. Random survival forests identify pathways with polymorphisms predictive of survival in KRAS mutant and KRAS wild-type metastatic colorectal cancer patients. Sci. Rep. 2021, 11, 12191. [Google Scholar] [CrossRef]
- He, Q.; Long, J.; Yin, Y.; Li, Y.; Lei, X.; Li, Z.; Zhu, W. Emerging Roles of lncRNAs in the Formation and Progression of Colorectal Cancer. Front. Oncol. 2020, 9, 1542. [Google Scholar] [CrossRef] [PubMed]
- De Bony, E.J.; Bizet, M.; van Grembergen, O.; Hassabi, B.; Calonne, E.; Putmans, P.; Bontempi, G.; Fuks, F. Comprehensive identification of long noncoding RNAs in colorectal cancer. Oncotarget 2018, 9, 27605–27629. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.F.; Stenvang, J.; Beck, M.K.; Hanáková, B.; Belling, K.C.; Do, K.N.; Viuff, B.; Nygård, S.B.; Gupta, R.; Rasmussen, M.H.; et al. Establishment and characterization of models of chemotherapy resistance in colorectal cancer: Towards a predictive signature of chemoresistance. Mol. Oncol. 2015, 9, 1169–1185. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Sakai, N.; Furukawa, K.; Takayashiki, T.; Kuboki, S.; Takano, S.; Ohira, G.; Miyauchi, H.; Matsubara, H.; Ohtsuka, M. Tumor-suppressive role of Smad ubiquitination regulatory factor 2 in patients with colorectal cancer. Sci. Rep. 2022, 12, 5495. [Google Scholar] [CrossRef]
- Li, J.; Galbo, P.M., Jr.; Gong, W.; Storey, A.J.; Tsai, Y.-H.; Yu, X.; Ahn, J.H.; Guo, Y.; Mackintosh, S.G.; Edmondson, R.D.; et al. ZMYND11-MBTD1 induces leukemogenesis through hijacking NuA4/TIP60 acetyltransferase complex and a PWWP-mediated chromatin association mechanism. Nat. Commun. 2021, 12, 1045. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, X.; Yin, J.; Dou, S.; Xie, X.; Liu, T.; Wang, Y.; Wang, S.; Zhou, X.; Zhang, D.; et al. RNF141 interacts with KRAS to promote colorectal cancer progression. Oncogene 2021, 40, 5829–5842. [Google Scholar] [CrossRef]
- Agrawal, N.; A Brown, M. Genetic associations and functional characterization of M1 aminopeptidases and immune-mediated diseases. Genes Immun. 2014, 15, 521–527. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, M.S.; Yoo, N.J.; Lee, S.H. BPTF, a chromatin remodeling-related gene, exhibits frameshift mutations in gastric and colorectal cancers. APMIS 2016, 124, 425–427. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, L.; Yu, D.; Zou, J.; Huang, Z. srGAP1 mediates the migration inhibition effect of Slit2-Robo1 in colorectal cancer. J. Exp. Clin. Cancer Res. 2016, 35, 191. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Navarrete, M.S.; Wang, Y.; McClintock, N.C.; Sakurai, R.; Wang, F.; Chen, K.T.; Chou, T.-F.; Rehan, V.K.; Lee, D.J.; et al. N-myristoyltransferase-1 is necessary for lysosomal degradation and mTORC1 activation in cancer cells. Sci. Rep. 2020, 10, 11952. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhuang, J.; Kang, D.; Chen, Y.; Song, W. Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis. Open Life Sci. 2021, 16, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; van Cutsem, E.; Lakomý, R.; Prausová, J.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.M.; Ferry, D.R.; McKendrick, J.J.; Soussan-Lazard, K.; et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: Prespecified subgroup analyses from the VELOUR trial. Eur. J. Cancer 2013, 50, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Avallone, A.; Nasti, G.; Rosati, G.; Carlomagno, C.; Romano, C.; Bilancia, D.; de Stefano, A.; Ottaiano, A.; Cassata, A.; Bianco, F.; et al. Optimization of the combination of bevacizumab with FOLFOX/OXXEL in patients with metastatic colorectal cancer (mCRC): The multicentre, randomized phase 3 study OBELICS. Ann. Oncol. 2017, 28, vi5. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Cornelio, G.; Shen, L.; Price, T.; Yang, T.-S.; Chung, I.J.; Dai, G.-H.; Lin, J.-K.; Sharma, A.; Yeh, K.-H.; et al. Efficacy, Tolerability, and Biomarker Analyses of Once-Every-2-Weeks Cetuximab Plus First-Line FOLFOX or FOLFIRI in Patients with KRAS or All RAS Wild-Type Metastatic Colorectal Cancer: The Phase 2 APEC Study. Clin. Color. Cancer 2016, 16, e73–e88. [Google Scholar] [CrossRef]
- Tabernero, J.; Ciardiello, F.; Rivera, F.; Rodriguez-Braun, E.; Ramos, F.J.; Martinelli, E.; Vega-Villegas, M.E.; Roselló, S.; Liebscher, S.; Kisker, O.; et al. Cetuximab administered once every second week to patients with metastatic colorectal cancer: A two-part pharmacokinetic/pharmacodynamic phase I dose-escalation study. Ann. Oncol. 2009, 21, 1537–1545. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Bondarenko, I.; Makhson, A.; Hartmann, J.T.; Aparicio, J.; de Braud, F.; Donea, S.; Ludwig, H.; Schuch, G.; Stroh, C.; et al. Fluorouracil, Leucovorin, and Oxaliplatin with and Without Cetuximab in the First-Line Treatment of Metastatic Colorectal Cancer. J. Clin. Oncol. 2009, 27, 663–671. [Google Scholar] [CrossRef]
- Siravegna, G.; Lazzari, L.; Crisafulli, G.; Sartore-Bianchi, A.; Mussolin, B.; Cassingena, A.; Martino, C.; Lanman, R.B.; Nagy, R.J.; Fairclough, S.; et al. Radiologic and Genomic Evolution of Individual Metastases during HER2 Blockade in Colorectal Cancer. Cancer Cell 2018, 34, 148–162.e7. [Google Scholar] [CrossRef]
- Smeets, D.; Miller, I.S.; O’Connor, D.P.; Das, S.; Moran, B.; Boeckx, B.; Gaiser, T.; Betge, J.; Barat, A.; Klinger, R.; et al. Copy number load predicts outcome of metastatic colorectal cancer patients receiving bevacizumab combination therapy. Nat. Commun. 2018, 9, 4112. [Google Scholar] [CrossRef]
- Seymour, M.T.; Brown, S.R.; Middleton, G.; Maughan, T.; Richman, S.; Gwyther, S.; Lowe, C.; Seligmann, J.F.; Wadsley, J.; Maisey, N.; et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): A prospectively stratified randomised trial. Lancet Oncol. 2013, 14, 749–759. [Google Scholar] [CrossRef]
- Tol, J.; Koopman, M.; Cats, A.; Rodenburg, C.J.; Creemers, G.J.; Schrama, J.G.; Erdkamp, F.L.; Vos, A.H.; van Groeningen, C.J.; Sinnige, H.A.; et al. Chemotherapy, Bevacizumab, and Cetuximab in Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 563–572. [Google Scholar] [CrossRef]
- Mondaca, S.; Walch, H.; Nandakumar, S.; Chatila, W.K.; Schultz, N.; Yaeger, R. Specific Mutations in APC, but Not Alterations in DNA Damage Response, Associate with Outcomes of Patients with Metastatic Colorectal Cancer. Gastroenterology 2020, 159, 1975–1978.e4. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Zanzotto, F.M.; Riondino, S.; Scarpato, N.; Guadagni, F.; Roselli, M. Breast Cancer Prognosis Using a Machine Learning Approach. Cancers 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Zanzotto, F.M.; Scarpato, N.; Riondino, S.; Guadagni, F.; Roselli, M. Validation of a Machine Learning Approach for Venous Thromboembolism Risk Prediction in Oncology. Dis. Markers 2017, 2017, 8781379. [Google Scholar] [CrossRef] [PubMed]
- Lindner, A.U.; Carberry, S.; Monsefi, N.; Barat, A.; Salvucci, M.; O’Byrne, R.; Zanella, E.R.; Cremona, M.; Hennessy, B.T.; Bertotti, A.; et al. Systems analysis of protein signatures predicting cetuximab responses in KRAS, NRAS, BRAF and PIK3CA wild-type patient-derived xenograft models of metastatic colorectal cancer. Int. J. Cancer 2020, 147, 2891–2901. [Google Scholar] [CrossRef]
- Agudo, A.; Peluso, M.; Munnia, A.; Luján-Barroso, L.; Barricarte, A.; Amiano, P.; Navarro, C.; Sánchez, M.-J.; Ardanaz, E.; Larrañaga, N.; et al. Aromatic DNA adducts and breast cancer risk: A case-cohort study within the EPIC-Spain. Carcinogenesis 2017, 38, 691–698. [Google Scholar] [CrossRef]
- Gilbertson, T.; Peluso, M.E.; Munia, A.; Luján-Barroso, L.; Sánchez, M.-J.; Navarro, C.; Amiano, P.; Barricarte, A.; Quirós, J.; Molina-Montes, E.; et al. Aromatic adducts and lung cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Spanish cohort. Carcinogenesis 2014, 35, 2047–2054. [Google Scholar] [CrossRef]
- Peluso, M.E.M.; Munnia, A.; Srivatanakul, P.; Jedpiyawongse, A.; Sangrajrang, S.; Ceppi, M.; Godschalk, R.W.; van Schooten, F.J.; Boffetta, P. DNA adducts and combinations of multiple lung cancer at-risk alleles in environmentally exposed and smoking subjects. Environ. Mol. Mutagen. 2013, 54, 375–383. [Google Scholar] [CrossRef]
- Peluso, M.E.M.; Munnia, A.; Bollati, V.; Srivatanakul, P.; Jedpiyawongse, A.; Sangrajrang, S.; Ceppi, M.; Giese, R.W.; Boffetta, P.; Baccarelli, A. Aberrant Methylation of Hypermethylated-in-Cancer-1 and Exocyclic DNA Adducts in Tobacco Smokers. Toxicol. Sci. 2013, 137, 47–54. [Google Scholar] [CrossRef]
- Peluso, M.; Bollati, V.; Munnia, A.; Srivatanakul, P.; Jedpiyawongse, A.; Sangrajrang, S.; Piro, S.; Ceppi, M.; Bertazzi, P.A.; Boffetta, P.; et al. DNA methylation differences in exposed workers and nearby residents of the Ma Ta Phut industrial estate, Rayong, Thailand. Int. J. Epidemiol. 2012, 41, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Chuang, S.-C.; Vaissiare, T.; Cuenin, C.; Ricceri, F.; Genair-EPIC Collaborators; Johansson, M.; Ueland, P.; Brennan, P.; Herceg, Z. DNA methylation changes associated with cancer risk factors and blood levels of vitamin metabolites in a prospective study. Epigenetics 2011, 6, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ching, T.; Himmelstein, D.S.; Beaulieu-Jones, B.K.; Kalinin, A.A.; Do, B.T.; Way, G.P.; Ferrero, E.; Agapow, P.-M.; Zietz, M.; Hoffman, M.M.; et al. Opportunities and obstacles for deep learning in biology and medicine. J. R. Soc. Interface 2018, 15, 20170387. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.P.; Jackson, R.; Dunne, D.F.J.; Malik, H.Z.; Fenwick, S.W.; Poston, G.J.; Ghaneh, P. Systematic review and meta-analysis of follow-up after hepatectomy for colorectal liver metastases. Br. J. Surg. 2012, 99, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Travers, A.; Jalali, A.; Begbie, S.; Semira, C.; Kosmider, S.; Ananda, S.; Wong, R.; Lee, M.; Shapiro, J.; Burge, M.; et al. Real-World Treatment and Outcomes of Metastatic Colorectal Cancer Patients with a Poor or Very Poor Performance Status. Clin. Color. Cancer 2020, 20, e21–e34. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Bagante, F.; Moris, D.; Cloyd, J.; Spartalis, E.; Pawlik, T.M. Clinical significance and prognostic relevance of KRAS, BRAF, PI3K and TP53 genetic mutation analysis for resectable and unresectable colorectal liver metastases: A systematic review of the current evidence. Surg. Oncol. 2018, 27, 280–288. [Google Scholar] [CrossRef]



| Problem Type | Measure | Explanation |
|---|---|---|
| Classification | AUC | Area under the receiver operating characteristic (ROC) curve. The ROC curve is created by plotting the true positive rate (TPR) against the false positive rate (FPR) at various discrimination threshold settings for binary classifiers. |
| SE | Sensitivity or true positive rate (TPR), is the fraction of actual positive cases that has been predicted as positive in the population. It is an evaluation measure for binary classifiers. | |
| SP | Specificity or true negative rate (TNR) refers to the fraction of true negative that has been predicted as negative in the population. It is an evaluation measure for binary classifier. | |
| ACC | Accuracy is the ratio of the number of correct predictions to the total number of input samples. | |
| PPV | Positive predictive value is the ratio of patients truly diagnosed as positive to all those who had positive test results. It is an evaluation measure for binary classifiers. | |
| NPV | Negative predictive value refers to the fraction of subjects truly diagnosed as negative among those who had negative test results. It is an evaluation measure for binary classifiers. | |
| Classification Precision | Same as PPV | |
| F1 score | It is defined as the harmonic mean of the precision and recall | |
| PLR | The Positive Likelihood Ratio is defined as the ratio between the true positivity rate and the false positivity rate. | |
| NLR | The Negative Likelihood Ratio is the opposite of the PLR. | |
| Regression\Survival Analysis | DFS | Disease-Free Survival is defined as the time from randomization to recurrence of tumor or death. |
| TTNT | Time-to-next treatment is defined as the time between baseline (randomization, inclusion or treatment initiation) and the date of subsequent systemic treatment initiation. | |
| C-index | Harrell’s concordance C-index: used to evaluate risk models in survival analysis. It is computed as the fraction of concordant patient pairs on the sum of concordant and discordant patient pairs, where two patients are considered concordant if the patient with the higher risk score is the one having a shorter time-to-disease. | |
| HR | In survival analysis, the hazard ratio is defined as the ratio of the hazard rates corresponding to the conditions described by two levels of an explanatory variable (e.g., cases vs. controls) |
| Therapy | AI | Total, Training, Test | Input Features | Signature | Evaluated Models and Selected Model | Training Set * | Test Set * | Reference |
|---|---|---|---|---|---|---|---|---|
| Imaging biomarkers learning models | ||||||||
| First-line FOLFOX chemotherapy | ML | 57 (242,172,70 features) | Contrast-enhanced computed tomography (CT) scans at differential time points 14 lesion shape-based 18 images intensity-based statistics 75 images gray level-based statistics | Delta-radiomics | Linear SVM Logistic regression Generalized linear Model with Poisson distribution Random Forest Decision Tree | Response vs. non-response: AUC = 0.99, 95% C.I. 0.97–1.0, 99% SE, 94% SP, 97% ACC, 95% PPV, 99% NPV | Response vs. non-response: AUC = 0.93, 95% C.I. 0.87–0.96, 85% SE, 92% SP, 86% ACC, 90% PPV, 97% NPV | [75] |
| First-line oxaliplatin-based regimens | ML | 42 (126,94,32 features) | Contrast-enhanced CT scans at baseline | Radiomics | Response vs. non-response: AUC = 0.851, 95% C.I. 0.771–0.930 | Response vs. non-response: AUC = 0.779, 95% C.I. 0.617–0.940 | [79] | |
| First-line CAPEOX or FOLFOX and FOLFIRI or XELIRI chemotherapies | DL | 192,144,48 (3490 features) | Contrast-enhanced multidetector tomography (MDCT) scans at baseline; 583 radiomics features (traditional radiomics) | Fusion radiomics | RESNET | Response vs. non-response: AUC = 0.903, 95% C.I. 0.851–0.955, 84.7% SE, 84.8% SP, 84.7% ACC (radiomics model) Response vs. non-response: AUC = 0.935, 95% C.I. 0.897–0.973, 89.8% SE, 84.8% SP, 88.2% ACC (fusion radiomics model) | Response vs. non-response: AUC = 0.745, 95% C.I. 0.659–0.831, 90% SE, 73% SP, 85.4% ACC (radiomics model) Response vs. non-response: AUC = 0.830, 95% C.I. 0.688–0.973, 90.9% SE, 73.3% SP, 85.4% ACC (fusion radiomics model) | [80] |
| First-line FOLFOX or FOLFIRI chemotherapies | ML | 92,54,38 | CT scans at baseline 75 radiomics features | Radiomics | Gaussian Naive Bayes Multilayer Perceptron Gaussian SVM | Response vs. non-response: 76% SE, 67% SP, 72% ACC, 69%, PPV, 75% NPV (per-lesion 3D set) | Response vs. non-response: 61% SE, 60% SP, 61% ACC, 57% PPV, 64% NPV (per-lesion 3D set) Response vs. non-response: 41% SE, 21% SP, 32% ACC, 38% PPV, 23% NPV (per-patient 3D set) | [81] |
| First-line FOLFIRI chemotherapy | ML | 129,78,51 (HQ set) 236,158,78 (SQ set) | High and standard quality (HQ and SQ sets) CT scans at differential time points 1749 Radiomics Features 1742 VGG16 extracted image features 8 rim specific features | Radiomics | For feature selection: RELIEF Fisher Score Chi Squared MRMR t-Test Wilcoxon Univariate models For classification: SVM KNN Naive Bayes Bagging Lasso Random Forest | Response vs. non-response: AUC = 0.75, 95% C.I. 0.63–0.85 (HQCT cohort) Response vs. non-response: AUC = 0.75, 95% C.I. 0.67–0.82 (SQCT cohort) | Response vs. non-response: AUC = 0.59, 95% C.I. 0.44–0.72 (HQCT cohort) Response vs. non-response: AUC = 0.55, 95% C.I. 0.43–0.66 (SQCT cohort) | [87] |
| Molecular biomarkers learning models | ||||||||
| First-line FOLFOX chemotherapy | ML | 115,83,32 | Microarray gene-expression data | 74 genes | Iterative supervised learning (IML) | Response vs. non-response: 97.6% SE, 100% SP | Response vs. non-response: OS = 13.4 vs. 36.6 mo HROS = 2.6 | [95] |
| First-line 5-fluorouracil-based regimens | ML | 2317,2277,40 | Immune infiltration data | 16 long noncoding RNA | Feature selection: Ensemble of statistical and machine learning models Survival analysis: Cox proportional hazard model | Response vs. non-response: AUC = 0.843 | [94] | |
| First-line 5-fluorouracil-based regimens | ML | 2307,2277,30 | Immune infiltration data | 16 long noncoding RNA | Feature selection: Ensemble of statistical and machine learning models Survival analysis: Cox proportional hazard model | Response vs. non-response: AUC = 0.765 | [94] | |
| First-line 5-fluorouracil-based regimens | ML | 2201,2277,124 | Immune infiltration data | 16 long noncoding RNA | Feature selection: Ensemble of statistical and machine learning models Survival analysis: Cox proportional hazard model | Response vs. non-response: AUC = 0.709 | [94] | |
| First-line FOLFIRI chemotherapy | ML | 82,27,55 | Next generation sequencing (NGS) data | 67 genes | SVM Logistic regression KNN Multilayer Perceptron Naive Bayes Quadratic Discriminant analysis Gaussian Process Random Forest Ensemble | Decreased survival: HROS = 2.631, 95% C.I. 1.041–6.649, OS = 18.7 vs. 34.4 mo | [99] | |
| First-line FOLFOX chemotherapy | ML | 83,54,29 | Microarray differentially expressed (DE) gene profiles | 18 genes | SVM KNN Gradient Boosting Machine Random Forest Decision Tree Neural Networks | Response vs. non-response: AUC = 0.877, 95% C.I. 0.747–1.00, 85% SE, 69.2% SP Improved survival: HROS = 0.358, 95% C.I. 0.178–0.717 in up-regulation of MLK1 Improved survival: HROS = 0.563, 95% C.I. 0.336–0.943 in up-regulation of CCDC124 | [101] | |
| First-line FOLFIRI chemotherapy | ML | 75,54,21 | Microarray DE gene profiles | 18 genes | SVM Neural Networks Random Forest | Response vs. non-response: AUC = 0.778, 95% C.I. 0.575–979 | [101] | |
| First-line oxaliplatin or irinotecan-based regimens | ML | 176,99,77 | Cytokine expression data | 17 cytokines | Feature selection: SVM Classification: Multiple linear regression | Response vs. non-response: 83.5% SE, 80% SP, 81% ACC, OS = 18.4 vs. 51.5 mo | Response vs. non-response: 83.1% SE, 66.7% SP, 74.9% ACC), OS = 16.8 vs. 55.9 mo | [83] |
| First-line FOLFOX chemotherapy | ML | 83,54,29 | Microarray DE gene profiles | 14 genes | Random Forest for feature selection, outlier detection and classification | Response vs. non-response: 91.3% SE, 95.6% SP, 80.2% ACC | Response vs. non-response: 80% SE, 92.8% SP, 69.2% ACC | [82] |
| First-line FOLFOX chemotherapy | ML | 44 | Protein pattern data | 6-proteins | Response vs. non-response: 92.9% SE, 81.3% SP | [84] | ||
| First-line FOLFIRI chemotherapy | ML | 26 | Protein pattern data | 7-proteins | Response vs. non-response: 92.3% SE, 92.3% SP | [84] | ||
| First-line FOLFIRI chemotherapy | ML | 21 | Microarray DE gene profiles | 14-genes | Response vs. non-response: 92% SE, 100% SP, 95% ACC | [92] | ||
| Clinical biomarkers learning models | ||||||||
| Irinotecan plus FOLFIRI chemotherapy | ML | 20 | Demographic data Liver function tests Tumor markers Pharmacokinetic parameters Anticancer treatment information; i.e., prior nonfuoropyrimidine regimen, prior surgery, prior radiation, fuoropyrimidine-based chemotherapy, concurrent surgery, concurrent radiation (categorical variables) | 3pharmacoparameters | Classification: BSLR: Backward Stepwise Logistic Regression (diarrhea) C4.5 algorithm Random Forest (leukopenia) SVM (neutropenia) | Toxic side-effect: AUC = 0.74, 89% SE, 60% SP, 76% ACC (leukopenia) Toxic side-effect: AUC = 0.88, 70% SE, 70% SP, 75% ACC (neutropenia) for toxic side-effect: AUC = 0.95, 81% SE, 100% SP = 100%, 91% ACC (diarrhea) | [93] | |
| First-line fluoropyrimidine-based regimens | ML | 36,030 patients (training set size not reported, used data from 2006-11 + 10-fold cross validation) (test set size not reported, used data from 2012-14) | Anticancer treatment information; i.e., prior nonfuoropyrimidine regimen, prior surgery, prior radiation, fuoropyrimidine-based chemotherapy, concurrent surgery, concurrent radiation -cancer information; i.e., cancer stage, tumor sizes demographic variables; i.e., age, sex, race, and geographic areas socioeconomic status; i.e., county-level median household income, education prior cardiovascular disease histories and other comorbidities; i.e., hypertension, hyperlipidemia, diabetes prior medications; i.e., beta-blockers, angiotensin-converting enzyme (ACE) inhibitors | Logistic Regression Random Forest XGBoost | Toxic side-effect: precision = 0.621, F1 = 0.396 AUC = 0.801, 95% C.I. 0.781–0.821 (cardiotoxicity) | [96] | ||
| Therapy | AI | Total, Training, Test | Input Features | Signature | Evaluated Models and Selected Model | Training Set | Test Set | Reference |
|---|---|---|---|---|---|---|---|---|
| Imaging biomarkers learning models | ||||||||
| Second-line anti-VEGF therapy (aflibercept plus FOLFIRI, prior bevacizumab plus oxaliplatin-based regimens) | DL | 1028,502,526 (3757,1864,1893 lesions) | Automatically extracted features from CT scans at differential time points | Radiomics | GoogleNet + LSTM with 4 times steps | Response vs. non-response: C-Index = 0.678, 95% 0.650–0.706 (DL model) | Response vs. non-response: C-IndexOS = 0.649, 95% C.I. 0.619–0.679 (DL model) C-IndexOS = 0.694, 95% C.I. 0.661–0.720 (DL model) OS = 18 vs. 10.4 mo (HR = 0.49, 95% C.I. 0.40–0.61) | [85] |
| First-line anti-VEGF therapy (bevacizumab plus oxaliplatin/fluoropyrimidine-based regimens) | ML | 76,52,24 | Magnetic resonance imaging (MRI) at baseline 48 texture features 15 morphological features | Radiomics | Features Selection: Lasso Classification:LDA SVM KNNArtificial neural network Decision tree | RAS mutation status and non-responder to anti-EGFR therapy: AUC = 0.79, 95% C.I. 0.70–0.85, 78% SE, 74.2% SP, 76.1% ACC (texture and morphological features model) Response vs. non-response according to RAS mutation status: AUC = 0.84, 95% C.I. 0.780–0.91, 90% SE, 67.8% SP, 76.9% ACC (texture features model) | RAS mutation status and non-responder to anti-EGFR therapy: 83.3% SE, 75% SP, 79.2% ACC (texture and morphological features model) 91.7% SE, 83.3% SP, 87.5% ACC (texture features model) | [91] |
| First-line anti-VEGF therapy (bevacizumab with or without OLFOX/FOLFIRI/XELOX, combined with anti-EGFR in KRAS, NRAS and BRAF wild-types) | DL | 180,101,79 (433,264,169 lesions) | MRI at differential time points | Radiomics | DC3CNN | Response vs. non-response: AUC = 0.849, 95% C.I. 0.737–0.926, 91.7% SE, 75% SP, ACC of 87.5%, 75% NPV, 91.7% PPV (four features model) Response vs. non-response: AUC = 0.833, 95% C.I. 0.695–1.000, 91.9% SE, 75% SP, ACC of 88.5%, 69.2% NPV, 93.8% PPV (four features model) | [98] | |
| \First-line anti-VEGF therapy (bevacizumab plus FOLFOX chemotherapy) | DL | 202,162,40 (treated/untreated lesion classification) 120,84,12,24 (treatment response classification) | Contrast-enhanced (CT) scans at baseline Texture analysis (TA) features | Radiomics | Treated/untreated classification: DT, SVM-RBF, ANN, Inception-inspired CNN Treatment response prediction: CNN | Response vs. non-response: AUC = 0.83, 95% C.I. 0.78–0.87, 97% SE, 59% SP, 78% ACC) | Response vs. non-response: AUC = 0.88, 95% C.I. 0.85–0.94, 98% SE, 54% SP, 76% ACC | [86] |
| First-line anti-EGFR therapy (cetuximab plus FOLFIRI chemotherapy) | ML | 116,78,38 (HQ set) 186,124,62 (SQ set) | High and standard quality (HQ and SQ) CT scans at differential time points 1749 Radiomics Features 1742 VGG16 extracted image features 8 rim specific features | Radiomics | For feature selection: RELIEF Fisher ScoreChi Squared MRMR t-Test Wilcoxon Univariate models For classification: SVM KNN Naive Bayes Bagging Lasso Random Forest | Response vs. non-response: AUC = 0.83, 95% C.I. 0.75–0.92, 77% SE, 85% SP (HQ cohort) AUC = 0.84, 95% C.I. 0.76–0.89 (SQ cohort) | Response vs. non-response: AUC = 0.80, 95% C.I. 0.69–0.94, 80% SE, 78% SP (HQ cohort) AUC = 0.72, 95% C.I. 0.59–0.83, 82% SE, 61% SP (SQ cohort) | [87] |
| First-line anti-EGFR therapy (cetuximab plus FOLFIRI or FOLFOX chemotherapies) | ML | 1886 | CT scans and MRI at baseline | Radiomics | Clustering: K-Means | Response vs. non-response in KRAS mutated tumors: HROS = 1.44, 95% C.I. 1.08–1.92 | [88] | |
| Last-line dual anti-HER2 therapy (lapatinib plus trastuzumab or pertuzumab plus trastuzumabemantansine) | ML | 38,28,10 patients 141,108,33 lesions | CT scans at baseline 24 radiomics features | Radiomics | Features Selection Genetic algorithms (GAs) ClassificationGaussian naïve Bayesian classifier | Response vs. non-response in RAS wild-type and HER2 amplified tumors: 89% SE, 85% SP, 93% PPV, 78% NPV (lesion model) | Response vs. non-response in RAS wild-type and HER2 amplified tumors: 90% SE, 42% SP, 73% PPV, 71% NPV (lesion model) 92% SE, 86% SP, 96% PPV, 75% NPV (patient model) | [74] |
| Molecular biomarkers learning models | ||||||||
| First-line anti-VEGF therapy (bevacizumab plus 5-fluorouracil-based regimens) | ML | 2289,2277,12 | Immune infiltration data | 16-long noncoding RNA | Feature selection: Ensemble of statistical and machine learning models Survival analysis: Cox proportional hazard model | Response vs. non-response: AUC = 0.771 | [94] | |
| First-line anti-VEGF therapy (bevacizumab plus 5-fluorouracil-based regimens) | ML | 2291,2277,14 | Immune infiltration data | 16-long noncoding RNA | Feature selection: Ensemble of statistical and machine learning models Survival analysis: Cox proportional hazard model | Response vs. non-response: AUC = 0.696 | [94] | |
| First-line anti-VEGF therapy (bevacizumab plus 5-fluorouracil-based regimens) | ML | 2305,2277,28 | Immune infiltration data | 16-long noncoding RNA | Feature selection: Ensemble of statistical and machine learning models Survival analysis: Cox proportional hazard model | Response vs. non-response: AUC = 0.781 | [94] | |
| First-line anti-VEGF therapy (bevacizumab plus FOLFOX chemotherapy) | ML | 650,105,545 | Next generation sequencing (NGS) data | 67-gene | SVM Logistic regression KNN Multilayer Perceptron Naive Bayes Quadratic Discriminant analysis Gaussian Process Random Forest Ensemble | Improved survival: HRTTNT = 0.537, 95% C.I. 0.428–0.674 TTNT = 11.5 vs. 8.2 mo Improved survival: HROS = 0.466, 95% C.I. 0.325–0.670 OS = 42 vs. 24.5 mo | Improved survival: HROS = 0.629, 95% C.I. 0.404–0.981 OS = 30 vs. 15.9 mo | [99] |
| First-line anti-VEGF therapy (bevacizumab plus FOLFOXIRI chemotherapy) | ML | 208,105,103 | NGS data | 67-gene | SVMLogistic regression KNN Multilayer Perceptron Naive Bayes Quadratic Discriminant analysis Gaussian Process Random Forest Ensemble | Improved survival: HROS = 0.483, 95% C.I. 0.270–0.864 OS = 30 vs. 15.9 mo | [99] | |
| First-line anti-VEGF therapy (bevacizumab plus FOLFIRI chemotherapy | ML | 488,345,143 | Genotyping data | 27 SNPs | Feature selection: Random Survival Forests Variable importance Minimal depth Survival analysis: Kaplan–Meier curves Log-rank test | Survival in KRAS wild-type with CBP rs129963 T/T variant: OS = 22.8 vs. 26 mo and PFS = 9.5 vs. 10.5 mo Survival in KRAS wild-type with TBK1 rs7486100 A/A variant: OS = 31.3 vs. 24.8 mo and PFS = 11.3 vs. 10.3 mo Survival in CCL2 rs4586 T/T carriers: OS = 30.9 vs. 22.8 mo Survival in VEGFR2 rs2305948 any C carriers: OS = 26.2 vs. 17.0 mo Survival in DMRT1 rs755383 any T carriers: PFS = 9.4 vs. 9.0 mo Survival in MMP2 rs243865 any T carriers: OS = 28.5 vs. 20.3 mo | Survival in KRAS mutant with β-catenin rs3864004 A/A genotype: OS = 16.3 vs. 26.3 mo and PFS = 7.8 vs. 9.6 mo Survival in KRAS mutant with TBK1 rs7486100 A/A variant: PFS = 10.3 vs. 8.6 mo | [102] |
| First-line anti-VEGF therapy (bevacizumab plus FOLFIRI chemotherapy) | ML | 558,180,378 | Exome-sequencing data | 1 or 2-SNPs | Combination of two Cox penalized regression models: Lasso Elastic Net | Improved survival: HRPFS = 0.52, 95% C.I. 0.33–0.83 Decreased survival: HRPFS = 2.3, 95% C.I. 1.19–4.57 Decreased survival in NLRP1 any A and SRL AA carriers: HRPFS = 8.3, 95% C.I. 3.3–21 and 2.2, 95% C.I. 1–5 Decreased survival in KRAS wild-type and concomitant carriers in combination with NLRP1 any A and SRL AA: HRPFS = 8.3, 95% C.I. 3.3–21 and 2.2, 95% C.I. 1–5 OS = 12 vs. 27 mo | Improved survival: HRPFS = 0.42, 95% C.I. 0.21–0.85 in the NLRP1 TT carriers Decreased survival: HRPFS = 2.5, 95% C.I. 1.12–5.5 in the SRL AA carrier | [89] |
| Second-line anti-EGFR therapy (panitumumab with or without irinotecan, prior fluoropyrimidine-based regimens, with or without oxaliplatin) | ML | 499,274,225 | Immunochemistry (IHC) data | Amphiregulin /epiregulin | Improved survival in KRAS wild-type: HRPFS = 0.54, 95% C.I. 0.37–0.79, OS = 8.0 vs. 3.2 mo Improved survival in KRAS and BRAF wild-types: HRPFS = 0.53, 95% C.I. 0.36–0.78 | [97] | ||
| First-line anti-EGFR therapy (CAPEOX-B chemotherapy plus or without cetuximab) | ML | 553,368,185 | Genome wide genotyping and survival data | 781-SNPs | Modified Random Forest using SurvDiff in place of Gini index to split the data at each node Classic RF with survival derived data labels | Response vs. non-response: HRbenefit = 0.69, 95% C.I. 0.49–0.98 Response vs. non-response: HRno benefit = 1.32, 95% C.I. 1.07–1.62 (SNP model) Response vs. non-response: HRbenefit = 0.52, 95% C.I. 0.35–0.76 (sex chromosome model) | [90] | |
| First-line anti-EGFR therapy (FOLFIRI) or anti-VEGF therapy (fluoropyrimidine-based regimens, with or without oxaliplatin or irinotecan or FOLFOXIRI or other regimens plus or without bevacizumab) | ML | 859,471,388 | NGS data | 7-gene classifier | Decreased survival: HRPFS = 16.9, 95% C.I. 4.2–68.0 | [100] | ||
| Study | ||||||
|---|---|---|---|---|---|---|
| State if Outliers with Impossible or Extreme Responses Are Removed; State Any Criteria Used for Outlier Removal. | State How Missing Values Were Handled. | External Validation Should also Be Performed Whenever Possible | If Possible, Report the Parameter Estimates in the Model and Their Confidence Intervals or Report Non-Parametric Estimates from Bootstrap Samples. | Meta-Analysis Inclusion | References | |
| Johnson (2022) | Yes | Yes | Yes | No | No | [100] |
| Li (2022) | Yes | Yes | Yes | Yes | No | [96] |
| Liu (2022) | No | No | Yes | No | No | [94] |
| Giannini (2022) | Yes | Yes | Yes | Yes | Yes | [75] |
| Granata (2021) | Yes | Yes | Yes | Yes | No | [91] |
| Abraham (2021) | No | No | Yes | Yes | Yes | [99] |
| Naseem (2021) | No | No | Yes | No | No | [102] |
| Defeudis (2021) | Yes | No | No | No | No | [81] |
| Nakanishi (2021) | Yes | Yes | No | Yes | Yes | [79] |
| Wei (2021) | No | No | No | No | Yes | [80] |
| Williams (2021) | No | No | No | Yes | No | [97] |
| Tian (2021) | Yes | Yes | Yes | Yes | No | [95] |
| Lu (2021) | No | No | No | Yes | No | [85] |
| Giannini (2020) | Yes | Yes | No | No | No | [74] |
| Ubels (2020) | No | No | No | Yes | Yes | [90] |
| Dercle (2020) | No | Yes | No | Yes | Yes | [87] |
| Barat (2020) | No | No | Yes | Yes | No | [89] |
| Zhu (2020) | No | Yes | Yes | Yes | Yes | [98] |
| Maaref (2020) | No | No | No | Yes | Yes | [86] |
| Lu (2020) | No | No | No | Yes | Yes | [101] |
| Vera-Yunca (2020) | Yes | No | No | Yes | No | [88] |
| Oyaga-Iriarte (2019) | No | No | No | No | No | [93] |
| Chen (2014) | No | No | No | Yes | No | [83] |
| Tsuji (2012) | Yes | Yes | No | No | No | [82] |
| Yuan (2012) | No | No | No | No | No | [84] |
| Del Rio (2007) | No | No | No | Yes | No | [92] |
| Study Included, Signature and Regimens | Performance Estimates | References | |
|---|---|---|---|
| Training sets | n | AUC, 95% C.I. | |
| Giannini (2022), radiomics signature and chemotherapy | 172 | 0.99, 95% C.I. 0.97–1.00 | [75] |
| Nakanishi (2021), radiomics signature and chemotherapy | 94 | 0.851, 95% C.I. 0.771–0.93 | [79] |
| Wei (2021), radiomics signature and chemotherapy | 144 | 0.935, 95% C.I. 0.897–0.973 | [80] |
| Dercle (2020), radiomics signature and chemotherapy | 78 | 0.75, 95% C.I. 0.63–0.85 | [87] |
| Dercle (2020), radiomics signature and targeted therapy | 78 | 0.83, 95% C.I. 0.75–0.92 | [87] |
| Maaref (2020), radiomics signature and targeted therapy | 162 | 0.83, 95% C.I. 0.78–0.87 | [86] |
| Validation sets | AUC, 95% C.I. | ||
| Giannini (2022), radiomics signature and chemotherapy | 70 | 0.93, 95% C.I. 0.87–0.96 | [75] |
| Nakanishi (2022), radiomics signature and chemotherapy | 32 | 0.779, 95% C.I. 0.617–0.94 | [79] |
| Wei (2021), radiomics signature and chemotherapy | 48 | 0.830, 95% C.I. 0.688–0.973 | [80] |
| Lu (2020), 18-gene signature and chemotherapy (FOLFOX) | 29 | 0.877, 95% C.I. 0.747–1.00 | [101] |
| Lu (2020) 18-gene signature and chemotherapy (FOLFIRI) | 21 | 0.778, 95% C.I. 0.575–0.979 | [101] |
| Dercle (2020), radiomics signature and chemotherapy | 51 | 0.59, 95% C.I. 0.44–0.72 | [87] |
| Dercle (2020), radiomics signature and targeted therapy | 38 | 0.80, 95% C.I. 0.69–0.94 | [87] |
| Zhu (2020), radiomics signature and targeted therapy | 79 | 0.849, 95% C.I. 0.737–0.926 | [98] |
| Zhu (2020), radiomics signature and targeted therapy | 73 | 0.833, 95% C.I. 0.695–1.00 | [98] |
| Maaref (2020), radiomics signature and targeted therapy | 40 | 0.88, 95% C.I. 0.85–0.94 | [86] |
| Validation sets | HR, 95% C.I. | ||
| Lu (2020), MLK1-gene signature and chemotherapy (FOLFOX) | 29 | 0.358, 95% C.I. 0.178–0.717 | [101] |
| Lu (2020), CCDC124-gene signature and chemotherapy (FOLFOX) | 29 | 0.563, 95% C.I. 336–0.943 | [101] |
| Lu (2021), radiomics learning models and targeted therapy | 526 | 0.49, 95% C.I. 0.4–0.61 | [85] |
| Abraham (2021), 67-gene signature and targeted therapy | 103 | 0.483, 95% C.I. 0.270–0.864 | [99] |
| Abraham (2021), 67-gene signature and targeted therapy | 545 | 0.629, 95% C.I. 0.404–0.981 | [99] |
| Therapy | AI | Signature | Training Set | Test Set | Reference |
|---|---|---|---|---|---|
| Chemotherapy | ML | Delta-radiomics | Excellent, 99% correctly classified as responders, 94% correctly classified as non-responders | Excellent, 85% correctly classified as responders, 92% correctly classified as non-responders | [75] |
| ML | Radiomics | Good | Poor | [79] | |
| DL | Fusion radiomics | Excellent, 84.7% correctly classified as responders, 84.8% correctly classified as non-responders (radiomics model) Excellent, 89.8% correctly classified as responders, 84.8% correctly classified as non-responders (fusion radiomics model) | Poor, 90% correctly classified as responders, 73% correctly classified as non-responders (radiomics model) Good, 90.9% correctly classified as responders, 73.3% correctly classified as non-responders (fusion radiomics model) | [80] | |
| ML | Radiomics | 76% correctly classified as responders, 67% correctly classified as non-responders (per-lesion 3D set) | 61% correctly classified as responders, 60% correctly classified as non-responders (per-lesion 3D set) 41% correctly classified as responders, 21% correctly classified as non-responders (per-patient 3D set) | [81] | |
| ML | Radiomics | Poor (high standard quality computed tomography scan, HQCT, set) Poor (high standard quality computed tomography scan, SQCT, set) | Failed (HQCT set) Failed (SQCT set) | [87] | |
| ML | 74 genes | 97.6% correctly classified as responders, 100% correctly classified as non-responders | [95] | ||
| ML | 16 long noncoding RNA | Good | [94] | ||
| ML | 16 long noncoding RNA | Poor | [94] | ||
| ML | 16 long noncoding RNA | Poor | [94] | ||
| Targeted therapy | DL | Radiomics | Good, 91.7% correctly classified as responders, 75% correctly classified as non-responders (four features model) Good, 91.9% correctly classified as responders, 75% correctly classified as non-responders (four features model) | [98] | |
| DL | Radiomics | Good, 97% correctly classified as responders, 59% correctly classified as non-responders | Good, 98% correctly classified as responders, 54% correctly classified as non-responders | [86] | |
| ML | Radiomics | Good, 77% correctly classified as responders, 85% correctly classified as non-responders (high quality, HQ, cohort) Good (standard quality, SQ, cohort) | Good, 80% correctly classified as responders, 78% correctly classified as non-responders (HQ cohort) Poor, 82% correctly classified as responders, 61% correctly classified as non-responders (SQ cohort) | [87] | |
| ML | Radiomics | 89% correctly classified as responders, 85% correctly classified as non-responders (lesion model) | 90% correctly classified as responders, 42% correctly classified as non-responders (lesion model) 92% correctly classified as responders, 86% correctly classified as non-responders (patient model) | [74] | |
| ML | 16-long noncoding RNA | Poor | [94] | ||
| ML | 16-long noncoding RNA | Worthless | [94] | ||
| ML | 16-long noncoding RNA | Poor | [94] | ||
| ML | 67-gene | Good | Poor | [99] | |
| Chemotherapy and targeted therapy | ML/DL | Imaging/molecular signatures | Good | Good | Present meta-analysis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, V.; Lallo, E.; Munnia, A.; Spedicato, M.; Messerini, L.; D’Aurizio, R.; Ceroni, E.G.; Brunelli, G.; Galvano, A.; Russo, A.; et al. Artificial Intelligence Predictive Models of Response to Cytotoxic Chemotherapy Alone or Combined to Targeted Therapy for Metastatic Colorectal Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4012. https://doi.org/10.3390/cancers14164012
Russo V, Lallo E, Munnia A, Spedicato M, Messerini L, D’Aurizio R, Ceroni EG, Brunelli G, Galvano A, Russo A, et al. Artificial Intelligence Predictive Models of Response to Cytotoxic Chemotherapy Alone or Combined to Targeted Therapy for Metastatic Colorectal Cancer Patients: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(16):4012. https://doi.org/10.3390/cancers14164012
Chicago/Turabian StyleRusso, Valentina, Eleonora Lallo, Armelle Munnia, Miriana Spedicato, Luca Messerini, Romina D’Aurizio, Elia Giuseppe Ceroni, Giulia Brunelli, Antonio Galvano, Antonio Russo, and et al. 2022. "Artificial Intelligence Predictive Models of Response to Cytotoxic Chemotherapy Alone or Combined to Targeted Therapy for Metastatic Colorectal Cancer Patients: A Systematic Review and Meta-Analysis" Cancers 14, no. 16: 4012. https://doi.org/10.3390/cancers14164012
APA StyleRusso, V., Lallo, E., Munnia, A., Spedicato, M., Messerini, L., D’Aurizio, R., Ceroni, E. G., Brunelli, G., Galvano, A., Russo, A., Landini, I., Nobili, S., Ceppi, M., Bruzzone, M., Cianchi, F., Staderini, F., Roselli, M., Riondino, S., Ferroni, P., ... Peluso, M. (2022). Artificial Intelligence Predictive Models of Response to Cytotoxic Chemotherapy Alone or Combined to Targeted Therapy for Metastatic Colorectal Cancer Patients: A Systematic Review and Meta-Analysis. Cancers, 14(16), 4012. https://doi.org/10.3390/cancers14164012