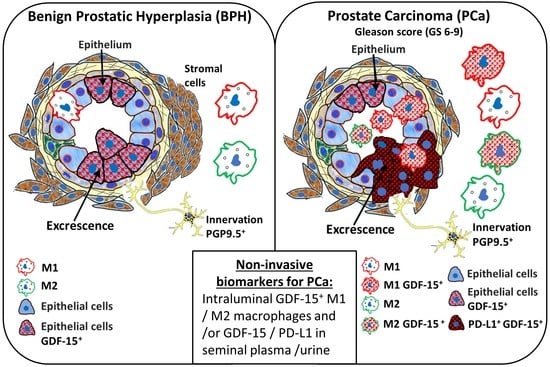
Increased Density of Growth Differentiation Factor-15+ Immunoreactive M1/M2 Macrophages in Prostate Cancer of Different Gleason Scores Compared with Benign Prostate Hyperplasia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Specimens and (Immuno) Histochemistry (IHC)
2.2. Double Immunofluorescence
2.3. Statistical Analyses
3. Results
3.1. The Changed PGP9.5+-Innervation Matrix in PCa of Different GS as Compared to BPH
3.2. Increased Density of GDF-15+ Cells in PCa of Different GS as Compared to BPH
3.3. Increased Density of CD68+ and CD163+ Macrophages in PCa of Different GS as Compared to BPH
3.4. Preferential Localization of GDF-15 in Transepithelial Migrating CD68+ or CD163+ MΦ and in PGP9.5+ Epithelial Cells in PCa
3.5. Relationship of GDF-15, PD-L1, CD68, and CD163 in Luminal Excrescences in PCa Compared to BPH
3.6. Decreased Density of CD4+ Lymphocytes in PCa Compared with BPH and Close Contact of CD19+ B Lymphocytes with Nerves in PCa but Not in BPH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Grozescu, T.; Popa, F. Prostate cancer between prognosis and adequate/proper therapy. J. Med. Life 2017, 10, 5–12. [Google Scholar] [PubMed]
- Humphrey, P.A. Histopathology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030411. [Google Scholar] [CrossRef]
- Epstein, J.I.; Amin, M.B.; Reuter, V.E.; Humphrey, P.A. Contemporary Gleason Grading of Prostatic Carcinoma: An Update With Discussion on Practical Issues to Implement the 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2017, 41, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Aponte-López, A.; Fuentes-Pananá, E.M.; Cortes-Muñoz, D.; Muñoz-Cruz, S. Mast Cell, the Neglected Member of the Tumor Microenvironment: Role in Breast Cancer. J. Immunol. Res. 2018, 2018, 2584243. [Google Scholar] [CrossRef]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Fukuoka, T.; Iseki, Y.; Matsutani, S.; Kashiwagi, S.; Tanaka, H.; Hirakawa, K.; Ohira, M. Tumor-infiltrating Lymphocytes Predict the Chemotherapeutic Outcomes in Patients with Stage IV Colorectal Cancer. In Vivo 2018, 32, 151–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Cai, D.L.; Jin, L.-P. Immune Cell Population in Ovarian Tumor Microenvironment. J. Cancer 2017, 8, 2915–2923. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, E.; Long, J.; Hu, Z.; Peng, J.; Liu, L.; Tang, F.; Li, L.; Ouyang, Y.; Zeng, Z. Immune infiltration in renal cell carcinoma. Cancer Sci. 2019, 110, 1564–1572. [Google Scholar] [CrossRef]
- Song, J.; Wang, W.; Yuan, Y.; Ban, Y.; Su, J.; Yuan, D.; Chen, W.; Zhu, J. Identification of immune-based prostate cancer subtypes using mRNA expression. Biosci. Rep. 2021, 41, BSR20201533. [Google Scholar] [CrossRef]
- Fairlie, W.; Moore, A.G.; Bauskin, A.R.; Russell, P.; Zhang, H.-P.; Breit, S.N. MIC-1 is a novel TGF-β superfamily cytokine associated with macrophage activation. J. Leukoc. Biol. 1999, 65, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Strelau, J.; Böttner, M.; Lingor, P.; Suter-Crazzolara, C.; Galter, D.; Jaszai, J.; Sullivan, A.; Schober, A.; Krieglstein, K.; Unsicker, K. GDF-15/MIC-1 a novel member of the TGF-ß superfamily. In Advances in Research on Neurodegeneration; Springer: Berlin, Germany, 2000; pp. 273–276. [Google Scholar] [CrossRef]
- Tsai, V.W.; Husaini, Y.; Sainsbury, A.; Brown, D.A.; Breit, S.N. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab. 2018, 28, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Pothuraju, R.; Khan, P.; Sharma, G.; Muniyan, S.; Seshacharyulu, P.; Jain, M.; Nasser, M.W.; Batra, S.K. Pathophysiological role of growth differentiation factor 15 (GDF15) in obesity, cancer, and cachexia. Cytokine Growth Factor Rev. 2022, 64, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Schober, A.; Böttner, M.; Strelau, J.; Kinscherf, R.; Bonaterra, G.A.; Barth, M.; Schilling, L.; Fairlie, W.; Breit, S.N.; Unsicker, K. Expression of growth differentiation factor-15/ macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in the perinatal, adult, and injured rat brain. J. Comp. Neurol. 2001, 439, 32–45. [Google Scholar] [CrossRef]
- Lockhart, S.M.; Saudek, V.; O’Rahilly, S. GDF15: A Hormone Conveying Somatic Distress to the Brain. Endocr. Rev. 2020, 41, bnaa007. [Google Scholar] [CrossRef] [PubMed]
- Paralkar, V.M.; Vail, A.L.; Grasser, W.A.; Brown, T.A.; Xu, H.; Vukicevic, S.; Ke, H.Z.; Qi, H.; Owen, T.A.; Thompson, D.D. Cloning and Characterization of a Novel Member of the Transforming Growth Factor-β/Bone Morphogenetic Protein Family. J. Biol. Chem. 1998, 273, 13760–13767. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.-H.; Chang, Y.-L.; Feng, T.-H.; Chung, L.-C.; Lee, T.-Y.; Chang, P.-L.; Juang, H.-H. Growth differentiation factor-15 upregulates interleukin-6 to promote tumorigenesis of prostate carcinoma PC-3 cells. J. Mol. Endocrinol. 2012, 49, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Spanopoulou, A.; Gkretsi, V. Growth differentiation factor 15 (GDF15) in cancer cell metastasis: From the cells to the patients. Clin. Exp. Metastasis 2020, 37, 451–464. [Google Scholar] [CrossRef]
- Welsh, J.B.; Sapinoso, L.M.; Kern, S.G.; Brown, D.A.; Liu, T.; Bauskin, A.R.; Ward, R.L.; Hawkins, N.J.; Quinn, D.I.; Russell, P.J.; et al. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc. Natl. Acad. Sci. USA 2003, 100, 3410–3415. [Google Scholar] [CrossRef]
- Li, J.; Veltri, R.W.; Yuan, Z.; Christudass, C.S.; Mandecki, W. Macrophage Inhibitory Cytokine 1 Biomarker Serum Immunoassay in Combination with PSA Is a More Specific Diagnostic Tool for Detection of Prostate Cancer. PLoS ONE 2015, 10, e0122249. [Google Scholar] [CrossRef]
- Brown, D.A.; Stephan, C.; Ward, R.L.; Law, M.; Hunter, M.; Bauskin, A.R.; Amin, J.; Jung, K.; Diamandis, E.P.; Hampton, G.M.; et al. Measurement of Serum Levels of Macrophage Inhibitory Cytokine 1 Combined with Prostate-Specific Antigen Improves Prostate Cancer Diagnosis. Clin. Cancer Res. 2006, 12, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Stephan, C.; Xu, C.; Brown, D.A.; Breit, S.N.; Michael, A.; Nakamura, T.; Diamandis, E.P.; Meyer, H.; Cammann, H.; Jung, K. Three new serum markers for prostate cancer detection within a percent free PSA-based artificial neural network. Prostate 2006, 66, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Johnen, H.; Lin, S.; Kuffner, T.; Brown, D.A.; Tsai, V.W.-W.; Bauskin, A.R.; Wu, L.; Pankhurst, G.; Jiang, L.; Junankar, S.; et al. Tumor-induced anorexia and weight loss are mediated by the TGF-β superfamily cytokine MIC-1. Nat. Med. 2007, 13, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, Z.; Fu, J.; Zhou, R. Growth and differentiation factor 15 regulates PD-L1 expression in glioblastoma. Cancer Manag. Res. 2019, 11, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zugazagoitia, J.; Ahmed, F.S.; Henick, B.S.; Gettinger, S.N.; Herbst, R.S.; Schalper, K.A.; Rimm, D.L. Immune Cell PD-L1 Colocalizes with Macrophages and Is Associated with Outcome in PD-1 Pathway Blockade Therapy. Clin. Cancer Res. 2020, 26, 970–977. [Google Scholar] [CrossRef]
- Lu, S.; Stein, J.E.; Rimm, D.L.; Wang, D.W.; Bell, J.M.; Johnson, D.B.; Sosman, J.A.; Schalper, K.A.; Anders, R.A.; Wang, H.; et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1195–1204. [Google Scholar] [CrossRef]
- Leonhardt, M.; Renneberg, H.; von Rahden, B.; Bjartell, A.; Abrahamsson, P.-A. Semiquantitative morphology of human prostatic development and regional distribution of prostatic neuroendocrine cells. Prostate 2001, 46, 108–115. [Google Scholar] [CrossRef]
- Szczyrba, J.; Niesen, A.; Wagner, M.; Wandernoth, P.M.; Aumüller, G.; Wennemuth, G. Neuroendocrine Cells of the Prostate Derive from the Neural Crest. J. Biol. Chem. 2017, 292, 2021–2031. [Google Scholar] [CrossRef]
- di Sant’Agnese, P.A.; Jensen, K.L.D.M.; Churukian, C.J.; Agarwal, M.M. Human prostatic endocrine-paracrine (APUD) cells. Distributional analysis with a comparison of serotonin and neuron-specific enolase immunoreactivity and silver stains. Arch. Pathol. Lab. Med. 1985, 109, 607–612. [Google Scholar]
- di Sant’Agnese, P.A. Neuroendocrine differentiation in carcinoma of the prostate. Diagnostic, prognostic, and therapeutic implications. Cancer 1992, 70, 254–268. [Google Scholar] [CrossRef]
- di Sant’Agnese, P.A. Neuroendocrine differentiation and prostatic carcinoma. The concept ‘comes of age’. Arch. Pathol. Lab. Med. 1988, 112, 1097–1099. [Google Scholar] [PubMed]
- Leiblich, A.; Cross, S.; Catto, J.; Pesce, G.; Hamdy, F.C.; Rehman, I. Human prostate cancer cells express neuroendocrine cell markers PGP 9.5 and chromogranin A. Prostate 2007, 67, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Fine, S.W. Neuroendocrine tumors of the prostate. Mod. Pathol. 2018, 31, 122–132. [Google Scholar] [CrossRef]
- Faulkner, S.; Jobling, P.; March, B.; Jiang, C.C.; Hondermarck, H. Tumor Neurobiology and the War of Nerves in Cancer. Cancer Discov. 2019, 9, 702–710. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Chen, N.; Niu, C.; Li, Z.; Hu, J.; Cui, J. Nerves in the Tumor Microenvironment: Origin and Effects. Front. Cell Dev. Biol. 2020, 8, 601738. [Google Scholar] [CrossRef]
- Sigorski, D.; Gulczyński, J.; Sejda, A.; Rogowski, W.; Iżycka-Świeszewska, E. Investigation of Neural Microenvironment in Prostate Cancer in Context of Neural Density, Perineural Invasion, and Neuroendocrine Profile of Tumors. Front. Oncol. 2021, 11, 710899. [Google Scholar] [CrossRef]
- March, B.; Faulkner, S.; Jobling, P.; Steigler, A.; Blatt, A.; Denham, J.; Hondermarck, H. Tumour innervation and neurosignalling in prostate cancer. Nat. Rev. Urol. 2020, 17, 119–130. [Google Scholar] [CrossRef]
- Rutledge, A.; Jobling, P.; Walker, M.M.; Denham, J.W.; Hondermarck, H. Spinal Cord Injuries and Nerve Dependence in Prostate Cancer. Trends Cancer 2017, 3, 812–815. [Google Scholar] [CrossRef]
- Hu, J.M.; Liu, K.; Liu, J.H.; Jiang, X.L.; Wang, X.L.; Chen, Y.Z.; Li, S.G.; Zou, H.; Pang, L.J.; Liu, C.X.; et al. CD163 as a marker of M2 macrophage, contribute to predict aggressiveness and prognosis of Kazakh esophageal squamous cell carcinoma. Oncotarget 2017, 8, 21526–21538. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Foster, D.S.; Jones, R.E.; Ransom, R.C.; Longaker, M.T.; Norton, J.A. The evolving relationship of wound healing and tumor stroma. JCI Insight 2018, 3, e99911. [Google Scholar] [CrossRef] [PubMed]
- Jobling, P.; Pundavela, J.; Oliveira, S.M.; Roselli, S.; Walker, M.M.; Hondermarck, H. Nerve–Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015, 75, 1777–1781. [Google Scholar] [CrossRef]
- Sejda, A.; Sigorski, D.; Gulczyński, J.; Wesołowski, W.; Kitlińska, J.; Iżycka-Świeszewska, E. Complexity of Neural Component of Tumor Microenvironment in Prostate Cancer. Pathobiology 2020, 87, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.; Doran, J.; Jackson, P.; Dhillon, A.; Rode, J. PGP 9.5—a new marker for vertebrate neurons and neuroendocrine cells. Brain Res. 1983, 278, 224–228. [Google Scholar] [CrossRef]
- Martìn, R.; Fraile, B.; Peinado, F.; Arenas, M.I.; Elices, M.; Alonso, L.; Paniagua, R.; Martìn, J.J.; Santamaráa, L. Immunohistochemical Localization of Protein Gene Product 9.5, Ubiquitin, and Neuropeptide Y Immunoreactivities in Epithelial and Neuroendocrine Cells from Normal and Hyperplastic Human Prostate. J. Histochem. Cytochem. 2000, 48, 1121–1130. [Google Scholar] [CrossRef]
- Hurst-Kennedy, J.; Chin, L.-S.; Li, L. Ubiquitin C-Terminal Hydrolase L1 in Tumorigenesis. Biochem. Res. Int. 2012, 2012, 123706. [Google Scholar] [CrossRef]
- Finnerty, B.M.; Moore, M.D.; Verma, A.; Aronova, A.; Huang, S.; Edwards, D.P.; Chen, Z.; Seandel, M.; Scognamiglio, T.; Du, Y.-C.N.; et al. UCHL1 loss alters the cell cycle in metastatic pancreatic neuroendocrine tumors. Endocrine-Related Cancer 2019, 26, 411–423. [Google Scholar] [CrossRef]
- Sadasivan, S.M.; Chen, Y.; Gupta, N.S.; Han, X.; Bobbitt, K.R.; Chitale, D.A.; Williamson, S.R.; Rundle, A.G.; Tang, D.; Rybicki, B.A. The interplay of growth differentiation factor 15 (GDF15) expression and M2 macrophages during prostate carcinogenesis. Carcinogenesis 2020, 41, 1074–1082. [Google Scholar] [CrossRef]
- Husaini, Y.; Tsai, V.W.-W.; Manandhar, R.; Zhang, H.P.; Lee-Ng, K.K.M.; Lebhar, H.; Marquis, C.P.; Brown, D.A.; Breit, S.N. Growth differentiation factor-15 slows the growth of murine prostate cancer by stimulating tumor immunity. PLoS ONE 2020, 15, e0233846. [Google Scholar] [CrossRef]
- Nakamura, T.; Scorilas, A.; Stephan, C.; Yousef, G.M.; Kristiansen, G.; Jung, K.; Diamandis, E.P. Quantitative analysis of macrophage inhibitory cytokine-1 (MIC-1) gene expression in human prostatic tissues. Br. J. Cancer 2003, 88, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Li, L.; Zhong, Q.; Rupp, N.J.; Charmpi, K.; E Wong, C.; Wagner, U.; Rueschoff, J.H.; Jochum, W.; Fankhauser, C.D.; et al. Multi-region proteome analysis quantifies spatial heterogeneity of prostate tissue biomarkers. Life Sci. Alliance 2018, 1, e201800042. [Google Scholar] [CrossRef] [PubMed]
- Vaňhara, P.; Hampl, A.; Kozubík, A.; Souček, K. Growth/differentiation factor-15: Prostate cancer suppressor or promoter? Prostate Cancer Prostatic Dis. 2012, 15, 320–328. [Google Scholar] [CrossRef]
- Wang, X.; Baek, S.J.; Eling, T.E. The diverse roles of nonsteroidal anti-inflammatory drug activated gene (NAG-1/GDF15) in cancer. Biochem. Pharmacol. 2013, 85, 597–606. [Google Scholar] [CrossRef]
- Lambert, J.R.; Whitson, R.J.; Iczkowski, K.A.; La Rosa, F.G.; Smith, M.L.; Wilson, R.S.; Smith, E.E.; Torkko, K.C.; Gari, H.H.; Lucia, M.S. Reduced expression of GDF-15 is associated with atrophic inflammatory lesions of the prostate. Prostate 2015, 75, 255–265. [Google Scholar] [CrossRef]
- Patrikainen, L.; Porvari, K.; Kurkela, R.; Hirvikoski, P.; Soini, Y.; Vihko, P. Expression profiling of PC-3 cell line variants and comparison of MIC-1 transcript levels in benign and malignant prostate. Eur. J. Clin. Investig. 2007, 37, 126–133. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Korets, L.V.; Coussens, L.M. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 2005, 7, 411–423. [Google Scholar] [CrossRef]
- Kerage, D.; Sloan, E.K.; Mattarollo, S.R.; McCombe, P.A. Interaction of neurotransmitters and neurochemicals with lymphocytes. J. Neuroimmunol. 2019, 332, 99–111. [Google Scholar] [CrossRef]
- Schmidt, M.; Böhm, D.; von Törne, C.; Steiner, E.; Puhl, A.; Pilch, H.; Lehr, H.A.; Hengstler, J.G.; Kölbl, H.; Gehrmann, M. The Humoral Immune System Has a Key Prognostic Impact in Node-Negative Breast Cancer. Cancer Res. 2008, 68, 5405–5413. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, H.Y.; Byun, Y.J.; Jeong, P.; Yoon, J.S.; Kim, D.H.; Kim, W.T.; Kim, Y.-J.; Lee, S.-C.; Yun, S.J.; et al. A novel urinary mRNA signature using the droplet digital polymerase chain reaction platform improves discrimination between prostate cancer and benign prostatic hyperplasia within the prostate-specific antigen gray zone. Investig. Clin. Urol. 2020, 61, 411–418. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yi, M.; Tan, L.; Huang, J.; Huang, L. The immune checkpoint regulator PD-L1 expression are associated with clinical progression in prostate cancer. World J. Surg. Oncol. 2021, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhu, Z.; Chen, X.; Zhang, H.; Huang, J.; Gu, S.; Zhao, X. The Importance of Exosomal PD-L1 in Cancer Progression and Its Potential as a Therapeutic Target. Cells 2021, 10, 3247. [Google Scholar] [CrossRef]
- Rubio-Briones, J.; Borque-Fernando, A.; Esteban, L.M.; Mascarós, J.M.; Ramírez-Backhaus, M.; Casanova, J.; Collado, A.; Mir, C.; Gómez-Ferrer, A.; Wong, A.; et al. Validation of a 2-gene mRNA urine test for the detection of ≥GG2 prostate cancer in an opportunistic screening population. Prostate 2020, 80, 500–507. [Google Scholar] [CrossRef]
- Lorente, G.; Ntostis, P.; Maitland, N.; Mengual, L.; Musquera, M.; Muneer, A.; Oliva, R.; Iles, D.; Miller, D. Semen sampling as a simple, noninvasive surrogate for prostate health screening. Syst. Biol. Reprod. Med. 2021, 67, 354–365. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonaterra, G.A.; Schleper, A.; Skowronek, M.; Kilian, L.S.; Rink, T.; Schwarzbach, H.; Heers, H.; Hänze, J.; Rexin, P.; Ramaswamy, A.; et al. Increased Density of Growth Differentiation Factor-15+ Immunoreactive M1/M2 Macrophages in Prostate Cancer of Different Gleason Scores Compared with Benign Prostate Hyperplasia. Cancers 2022, 14, 4591. https://doi.org/10.3390/cancers14194591
Bonaterra GA, Schleper A, Skowronek M, Kilian LS, Rink T, Schwarzbach H, Heers H, Hänze J, Rexin P, Ramaswamy A, et al. Increased Density of Growth Differentiation Factor-15+ Immunoreactive M1/M2 Macrophages in Prostate Cancer of Different Gleason Scores Compared with Benign Prostate Hyperplasia. Cancers. 2022; 14(19):4591. https://doi.org/10.3390/cancers14194591
Chicago/Turabian StyleBonaterra, Gabriel A., Alexander Schleper, Maximilian Skowronek, Lucia S. Kilian, Theresa Rink, Hans Schwarzbach, Hendrik Heers, Jörg Hänze, Peter Rexin, Annette Ramaswamy, and et al. 2022. "Increased Density of Growth Differentiation Factor-15+ Immunoreactive M1/M2 Macrophages in Prostate Cancer of Different Gleason Scores Compared with Benign Prostate Hyperplasia" Cancers 14, no. 19: 4591. https://doi.org/10.3390/cancers14194591
APA StyleBonaterra, G. A., Schleper, A., Skowronek, M., Kilian, L. S., Rink, T., Schwarzbach, H., Heers, H., Hänze, J., Rexin, P., Ramaswamy, A., Denkert, C., Wilhelm, B., Hegele, A., Hofmann, R., Weihe, E., & Kinscherf, R. (2022). Increased Density of Growth Differentiation Factor-15+ Immunoreactive M1/M2 Macrophages in Prostate Cancer of Different Gleason Scores Compared with Benign Prostate Hyperplasia. Cancers, 14(19), 4591. https://doi.org/10.3390/cancers14194591