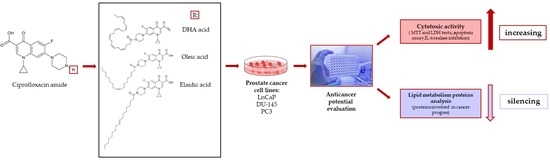
The Effect of Fatty Acids on Ciprofloxacin Cytotoxic Activity in Prostate Cancer Cell Lines—Does Lipid Component Enhance Anticancer Ciprofloxacin Potential?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Ciprofloxacin Derivatives
2.1.2. Cell Culture
2.2. Methods
2.2.1. MTT Assay
2.2.2. LDH Test
2.2.3. Apoptosis Detection
2.2.4. Interleukin-6 Assay
2.2.5. Proteomic Analysis
2.2.6. Statistical Analysis
3. Results
3.1. Cytotoxic Activity
3.1.1. MTT Assay
- Effect of conjugated ciprofloxacin with fatty acids
- Effect of unconjugated ciprofloxacin and fatty acids
3.1.2. LDH Assay
- Effect of conjugated ciprofloxacin with fatty acids
- Effect of unconjugated ciprofloxacin and fatty acids
3.2. Mechanism of Conjugates Cytotoxicity
3.2.1. Apoptosis
3.2.2. IL-6 Release
4. A Summary of the Cancer Cell Lines Sensitivity to Individual Conjugates
5. Proteomic Analysis
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCa | prostate cancer |
| CRPC | castration-resistant PCa |
| CP | Ciprofloxacin |
| FA | fatty acid |
| LDs | lipid droplets |
| AR | androgen receptor |
| IL-6 | interleukin 6 |
| OA | oleic acid |
| EA | elaidic acid |
| DHA | docosahexaenoic acid |
| NF-κB | nuclear factor-κB |
| FITC | fluorescin |
| EGFR | epidermal growth factor receptor |
References
- Leslie, S.W.; Soon-Sutton, T.L.; Sajjad, H.; Siref, L.E. Prostate Cancer; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Saraon, P.; Drabovich, A.P.; Jarvi, K.A.; Diamandis, E.P. Mechanisms of Androgen-Independent Prostate Cancer. EJIFCC 2014, 25, 42–54. [Google Scholar] [PubMed]
- Sharma, P.C.; Jain, A.; Jain, S.; Pahwa, R.; Yar, M.S. Ciprofloxacin: Review on developments in synthetic, analytical, and medicinal aspects. J. Enzyme Inhib. Med. Chem. 2010, 25, 577–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hangas, A.; Aasumets, K.; Kekalainen, N.J.; Paloheina, M.; Pohjoismaki, J.L.; Gerhold, J.M.; Goffart, S. Ciprofloxacin impairs mitochondrial DNA replication initiation through inhibition of Topoisomerase 2. Nucleic Acids Res. 2018, 46, 9625–9636. [Google Scholar] [CrossRef]
- Aranha, O.; Grignon, R.; Fernandes, N.; McDonnell, T.J.; Wood, D.P., Jr.; Sarkar, F.H. Suppression of human prostate cancer cell growth by ciprofloxacin is associated with cell cycle arrest and apoptosis. Int. J. Oncol. 2003, 22, 787–794. [Google Scholar] [PubMed]
- Pinto, A.C.; Moreira, J.N.; Simoes, S. Ciprofloxacin sensitizes hormone-refractory prostate cancer cell lines to doxorubicin and docetaxel treatment on a schedule-dependent manner. Cancer Chemother. Pharmacol. 2009, 64, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raub, T.J. P-glycoprotein recognition of substrates and circumvention through rational drug design. Mol. Pharm. 2006, 3, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, M.; Filipowska, A.; Fiorino, F.; Struga, M. Anticancer activities of fatty acids and their heterocyclic derivatives. Eur. J. Pharmacol. 2020, 871, 172937. [Google Scholar] [CrossRef]
- Peters, G.J.; Voorn, D.A.; Kuiper, C.M.; van der Wilt, C.L.; Noordhuis, P.; Smid, K.; Myhren, F.; Sandvold, M.; Hendriks, H.R. Cell specific cytotoxicity and structure-activity relationship of lipophilic 1-B-D-arabinofuranosylcytosine (ara-C) derivatives. Nucleosides Nucleotides 1999, 18, 877–878. [Google Scholar] [CrossRef] [PubMed]
- Zaro, J.L. Lipid-based drug carriers for prodrugs to enhance drug delivery. AAPS J. 2015, 17, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Alanazi, A.M.; Jabeen, M.; Chauhan, A.; Abdelhameed, A.S. Design, synthesis and in vitro anticancer evaluation of a stearic acid-based ester conjugate. Anticancer Res. 2013, 33, 2517–2524. [Google Scholar]
- Kaighn, M.E.; Lechner, J.F.; Narayan, K.S.; Jones, L.W. Prostate carcinoma: Tissue culture cell lines. Natl. Cancer Inst. Monogr. 1978, 49, 17–21. [Google Scholar]
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer 1978, 21, 274–281. [Google Scholar] [CrossRef]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar]
- Bello, D.; Webber, M.M.; Kleinman, H.K.; Wartinger, D.D.; Rhim, J.S. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis 1997, 18, 1215–1223. [Google Scholar] [CrossRef]
- Wu, X.; Daniels, G.; Lee, P.; Monaco, M.E. Lipid metabolism in prostate cancer. Am. J. Clin. Exp. Urol. 2014, 2, 111–120. [Google Scholar] [PubMed]
- Zaidi, N.; Lupien, L.; Kuemmerle, N.B.; Kinlaw, W.B.; Swinnen, J.V.; Smans, K. Lipogenesis and lipolysis: The pathways exploited by the cancer cells to acquire fatty acids. Prog. Lipid Res. 2013, 52, 585–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, I.J.; Eckel, R.H.; Abumrad, N.A. Regulation of fattty acid uptake into tissues: Lipoprotein lipase- and CD36-mediated pathways. J. Lipid Res. 2009, 50, S86–S90. [Google Scholar] [CrossRef] [Green Version]
- Dang, Q.; Chen, Y.A.; Hsieh, J.T. The dysfunctional lipids in prostate cancer. Am. J. Clin. Exp. Urol. 2019, 7, 273–280. [Google Scholar] [PubMed]
- Roman, M.; Wrobel, T.P.; Panek, A.; Paluszkiewicz, C.; Kwiatek, W.M. Lipid droplets in prostate cancer cells and effect of irradiation studied by Raman microspectroscopy. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158753. [Google Scholar] [CrossRef]
- Stoykova, G.E.; Schlaepfer, I.R. Lipid Metabolism and Endocrine Resistance in Prostate Cancer, and New Opportunities for Therapy. Int. J. Mol. Sci. 2019, 20, 2626. [Google Scholar] [CrossRef] [Green Version]
- Chrzanowska, A.; Roszkowski, P.; Bielenica, A.; Olejarz, W.; Stepien, K.; Struga, M. Anticancer and antimicrobial effects of novel ciprofloxacin fatty acids conjugates. Eur. J. Med. Chem. 2020, 185, 111810. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blazquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095505. [Google Scholar] [CrossRef]
- Duensing, T.D.; Watson, S.R. Assessment of Apoptosis (Programmed Cell Death) by Flow Cytometry. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot093807. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Talwar, P. Repositioning of fluoroquinolones from antibiotic to anti-cancer agents: An underestimated truth. Biomed. Pharmacother. 2019, 111, 934–946. [Google Scholar] [CrossRef]
- Aranha, O.; Wood, D.P., Jr.; Sarkar, F.H. Ciprofloxacin mediated cell growth inhibition, S/G2-M cell cycle arrest, and apoptosis in a human transitional cell carcinoma of the bladder cell line. Clin. Cancer Res. 2000, 6, 891–900. [Google Scholar]
- Kloskowski, T.; Szeliski, K.; Fekner, Z.; Rasmus, M.; Dabrowski, P.; Wolska, A.; Siedlecka, N.; Adamowicz, J.; Drewa, T.; Pokrywczynska, M. Ciprofloxacin and Levofloxacin as Potential Drugs in Genitourinary Cancer Treatment-The Effect of Dose-Response on 2D and 3D Cell Cultures. Int. J. Mol. Sci. 2021, 22, 11970. [Google Scholar] [CrossRef]
- Kuemmerle, N.B.; Rysman, E.; Lombardo, P.S.; Flanagan, A.J.; Lipe, B.C.; Wells, W.A.; Pettus, J.R.; Froehlich, H.M.; Memoli, V.A.; Morganelli, P.M.; et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol. Cancer Ther. 2011, 10, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Gazi, E.; Gardner, P.; Lockyer, N.P.; Hart, C.A.; Brown, M.D.; Clarke, N.W. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. J. Lipid Res. 2007, 48, 1846–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertilsson, H.; Tessem, M.B.; Flatberg, A.; Viset, T.; Gribbestad, I.; Angelsen, A.; Halgunset, J. Changes in gene transcription underlying the aberrant citrate and choline metabolism in human prostate cancer samples. Clin. Cancer Res. 2012, 18, 3261–3269. [Google Scholar] [CrossRef] [Green Version]
- Yue, S.; Li, J.; Lee, S.Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef] [Green Version]
- Simplicio, A.L.; Clancy, J.M.; Gilmer, J.F. Prodrugs for amines. Molecules 2008, 13, 519–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brueckner, B.; Rius, M.; Markelova, M.R.; Fichtner, I.; Hals, P.A.; Sandvold, M.L.; Lyko, F. Delivery of 5-azacytidine to human cancer cells by elaidic acid esterification increases therapeutic drug efficacy. Mol. Cancer Ther. 2010, 9, 1256–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmori, H.; Fujii, K.; Kadochi, Y.; Mori, S.; Nishiguchi, Y.; Fujiwara, R.; Kishi, S.; Sasaki, T.; Kuniyasu, H. Elaidic Acid, a Trans-Fatty Acid, Enhances the Metastasis of Colorectal Cancer Cells. Pathobiology 2017, 84, 144–151. [Google Scholar] [CrossRef]
- Kishi, S.; Fujiwara-Tani, R.; Luo, Y.; Kawahara, I.; Goto, K.; Fujii, K.; Ohmori, H.; Nakashima, C.; Sasaki, T.; Kuniyasu, H. Pro-metastatic signaling of the trans fatty acid elaidic acid is associated with lipid rafts. Oncol. Lett. 2018, 15, 4423–4426. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, I.A.; Brown, I.; Schofield, A.C.; Wahle, K.W.; Heys, S.D. Docosahexaenoic acid enhances the efficacy of docetaxel in prostate cancer cells by modulation of apoptosis: The role of genes associated with the NF-kappaB pathway. Prostate 2008, 68, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Oono, K.; Takahashi, K.; Sukehara, S.; Kurosawa, H.; Matsumura, T.; Taniguchi, S.; Ohta, S. Inhibition of PC3 human prostate cancer cell proliferation, invasion and migration by eicosapentaenoic acid and docosahexaenoic acid. Mol. Clin. Oncol. 2017, 7, 217–220. [Google Scholar] [CrossRef] [Green Version]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Newell, M.; Baker, K.; Postovit, L.M.; Field, C.J. A Critical Review on the Effect of Docosahexaenoic Acid (DHA) on Cancer Cell Cycle Progression. Int. J. Mol. Sci. 2017, 18, 1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grasso, P.; Gangolli, S.D.; Hooson, J. Connective tissue response to a short-term series of subcutaneous injections of sorbic acid or aflatoxin. Physico-chemical factors determining reaction to sorbic acid. Br. J. Cancer 1969, 23, 787–799. [Google Scholar] [CrossRef] [Green Version]
- Dickens, F.; Jones, H.E.; Waynforth, H.B. Further tests on the carcinogenicity of sorbic acid in the rat. Br. J. Cancer 1968, 22, 762–768. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Kume, H.; Matsuzaki, K.; Kawashima, A.; Ujike, T.; Nagahara, A.; Uemura, M.; Miyagawa, Y.; Tomonaga, T.; Nonomura, N. Proteomic analysis of urinary extracellular vesicles from high Gleason score prostate cancer. Sci. Rep. 2017, 7, 42961. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.A.; Quazi, F.; Molday, R.S. Mammalian P4-ATPases and ABC transporters and their role in phospholipid transport. Biochim. Biophys. Acta 2013, 1831, 555–574. [Google Scholar] [CrossRef] [Green Version]
- Cruz, P.M.; Mo, H.; McConathy, W.J.; Sabnis, N.; Lacko, A.G. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: A review of scientific findings, relevant to future cancer therapeutics. Front. Pharmacol. 2013, 4, 119. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.I.; Diaz, P.; Bennett, J.C.; Urra, H.; Ortiz, R.; Orellana, P.C.; Hetz, C.; Quest, A.F.G. Caveolin-1 suppresses tumor formation through the inhibition of the unfolded protein response. Cell Death Dis. 2020, 11, 648. [Google Scholar] [CrossRef]
- Thompson, T.C.; Tahir, S.A.; Li, L.; Watanabe, M.; Naruishi, K.; Yang, G.; Kadmon, D.; Logothetis, C.J.; Troncoso, P.; Ren, C.; et al. The role of caveolin-1 in prostate cancer: Clinical implications. Prostate Cancer Prostatic Dis. 2010, 13, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, V.; Benfodda, Z.; Rodier, G.; Henriquet, C.; Iborra, F.; Avances, C.; Allory, Y.; de la Taille, A.; Culine, S.; Blancou, H.; et al. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol. Cancer Ther. 2010, 9, 1740–1754. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Rajput, S.; Watabe, K.; Liao, D.F.; Cao, D. Acetyl-CoA carboxylase-a as a novel target for cancer therapy. Front. Biosci. 2010, 2, 515–526. [Google Scholar] [CrossRef]
- Ishizaki, F.; Nishiyama, T.; Kawasaki, T.; Miyashiro, Y.; Hara, N.; Takizawa, I.; Naito, M.; Takahashi, K. Androgen deprivation promotes intratumoral synthesis of dihydrotestosterone from androgen metabolites in prostate cancer. Sci. Rep. 2013, 3, 1528. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Chen, S.; Ng, P.; Bubley, G.J.; Nelson, P.S.; Mostaghel, E.A.; Marck, B.; Matsumoto, A.M.; Simon, N.I.; Wang, H.; et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011, 71, 6503–6513. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Lin, R.; Xia, S.; Chen, D.; Elf, S.E.; Liu, S.; Pan, Y.; Xu, H.; Qian, Z.; Wang, M.; et al. Tetrameric Acetyl-CoA Acetyltransferase 1 Is Important for Tumor Growth. Mol. Cell 2016, 64, 859–874. [Google Scholar] [CrossRef] [Green Version]
- Ursini-Siegel, J.; Rajput, A.B.; Lu, H.; Sanguin-Gendreau, V.; Zuo, D.; Papavasiliou, V.; Lavoie, C.; Turpin, J.; Cianflone, K.; Huntsman, D.G.; et al. Elevated expression of DecR1 impairs ErbB2/Neu-induced mammary tumor development. Mol. Cell Biol. 2007, 27, 6361–6371. [Google Scholar] [CrossRef] [Green Version]
- Blomme, A.; Ford, C.A.; Mui, E.; Patel, R.; Ntala, C.; Jamieson, L.E.; Planque, M.; McGregor, G.H.; Peixoto, P.; Hervouet, E.; et al. 2,4-dienoyl-CoA reductase regulates lipid homeostasis in treatment-resistant prostate cancer. Nat. Commun. 2020, 11, 2508. [Google Scholar] [CrossRef] [PubMed]






| No | Substituent (R) | The Substituent Name (Common/IUPAC) | Chain Length/Number of Unsaturation/Isomer |
|---|---|---|---|
| 1 | C3H5C(O)- | Crotonic acid (2E)-But-2-enoic acid | 4:1 (E2) |
| 2 | C5H7C(O)- | Sorbic acid (2E,4E)-hexa-2,4-dienoic acid | 6:2 (E2, E4) |
| 3 | C9H15C(O)- | Geranic acid (2E)-3,7-Dimethyl-2,6-octadienoic acid | 10:2 (E2, E6) |
| 4 | C17H33C(O)- | Oleic acid (9Z)-Octadec-9-enoic acid | 18:1 (Z9) |
| 5 | C17H33C(O)- | Elaidic acid (E)-octadec-9-enoic acid | 18:1 (E9) |
| 6 | C17H29C(O)- | Linolenic acid (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid | 18:3 (Z9, Z12, Z15) |
| 7 | C21H41C(O)- | Erucic acid (Z)-Docos-13-enoic acid | 22:1 (Z13) |
| 8 | C21H31C(O)- | Docosahexaenoic acid (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid | 22:6 (Z4, Z7, Z10, Z13, Z16, Z19) |
| 9 | C15H31C(O)- | Palmitic acid Hexadecanoic acid | 16:0 |
| Compound | Cancer Cells | Normal Cells | |||||
|---|---|---|---|---|---|---|---|
| LNCaP c | DU-145 d | PC3 e | RWPE-1 f | ||||
| IC50 a | SI b | IC50 | SI | IC50 | SI | IC50 | |
| 1 | 66.7 ± 7.9 | 1.4 | 62.4 ± 5.6 | 1.4 | 93.8 ± 3.4 | 0.9 | 81.2 ± 6.9 |
| 2 | 38.8 ± 3.2 | 1.6 | 24.7 ± 5.3 | 2.5 | 11.7 ± 1.8 | 5.3 | 62.5 ± 6.4 |
| 3 | 39.5 ± 3.1 | 1.9 | 33.8 ± 2.1 | 2.3 | 73.4 ± 2.7 | 1.1 | 78.1 ± 3.6 |
| 4 | 24.7 ± 4.1 | 2.7 | 20.2 ± 1.9 | 3.3 | 7.7 ± 2.1 | 8.7 | 66.9 ± 3.8 |
| 5 | 22.4 ± 3.1 | 2.9 | 17.8 ± 1.6 | 3.6 | 15.3 ± 5.3 | 4.2 | 64.8 ± 5.9 |
| 6 | 39.8 ± 4.6 | 1.4 | 25.9 ± 2.4 | 1.8 | 34.4 ± 2.4 | 1.7 | 57.2 ± 6.7 |
| 7 | 59.2 ± 4.3 | 1.0 | 27.6 ± 2.4 | 2.2 | 76.3 ± 3.3 | 0.8 | 60.8 ± 5.3 |
| 8 | 21.4 ± 2.2 | 3.0 | 16.5 ± 1.4 | 4 | 27.7 ± 1.9 | 2.3 | 65.1 ± 2.2 |
| 9 | 39.0 ± 4.7 | 1.3 | 48.1 ± 2.3 | 1.1 | 51.08 ± 4.5 | 1.0 | 51.3 ± 4.6 |
| Ciprofloxacin g | 71.2 ± 3.8 | 1.0 | 70.5 ± 3.6 | 1.0 | 101.38 ± 3.6 | 0.7 | 72.1 ± 5.2 |
| Cisplatin h | 1.78 ± 0.74 | 1.5 | 1.36 ± 0.69 | 2 | 1.51 ± 0.24 | 1.78 | 2.69 ± 1.1 |
| Doxorubicin h | 0.48 ± 0.21 | 1.3 | 0.59 ± 0.14 | 1.1 | 0.31 ± 0.12 | 2 | 0.65 ± 0.1 |
| Compounds | Cancer Cells | Normal Cells | |||||
|---|---|---|---|---|---|---|---|
| LNCaP c | DU145 d | PC3 e | RWPE-1 f | ||||
| IC50 a | SI b | IC50 | SI | IC50 | SI | IC50 | |
| CA g + CP h | 65.6 ± 6.9 | 1.1 | 75.2 ± 4.8 | 0.9 | 79.2 ± 3.4 | 0.9 | 73.1 ± 5.7 |
| SA + CP | 69.1 ± 7.4 | 1.0 | 69.3 ± 7.3 | 1.0 | 68.4 ± 2.8 | 1.1 | 73.2 ± 9.5 |
| GA + CP | 69.3 ± 8.5 | 0.9 | 76.1 ± 8.3 | 0.9 | 72.2 ± 5.8 | 0.9 | 66.3 ± 9.2 |
| OA + CP | 51.8 ± 4.9 | 1.2 | 50.7 ± 4.8 | 0.9 | 67.3 ± 4.7 | 0.9 | 60.2 ± 9.3 |
| EA + CP | 37.7 ± 5.5 | 1.2 | 31.4 ± 5.2 | 1.4 | 66.3 ± 5.1 | 0.7 | 46.3 ± 5.9 |
| LA + CP | 61.7 ± 7.3 | 0.9 | 51.6 ± 4.9 | 1.1 | 58.2 ± 3.5 | 1.0 | 55.9 ± 6.7 |
| EA + CP | 54.2 ± 6.5 | 1.1 | 58.6 ± 6.5 | 1.0 | 79.3 ± 6.2 | 0.8 | 61.6 ± 8.4 |
| DHA + CP | 38.4 ± 4.7 | 1.3 | 36.9 ± 3.7 | 1.3 | 43.5 ± 3.7 | 1.1 | 49.7 ± 6.3 |
| PA + CP | 37.7 ± 9.7 | 1.3 | 42.5 ± 6.4 | 1.1 | 61.23 ± 4.2 | 0.8 | 49.3 ± 5.8 |
| CP | 71.2 ± 3.8 | 1.0 | 70.5 ± 3.6 | 1.0 | 101.38 ± 3.6 | 0.7 | 72.1 ± 5.2 |
| Cell Line | Compound | Early Apoptosis a (%) | SD | Late apoptosis b (%) | SD |
|---|---|---|---|---|---|
| Control | - | 0.2 | 0.1 | 0.2 | 0.1 |
| RWPE-1 | 4 | 0.8 | 0.3 | 1.3 | 0.7 |
| 5 | 1.3 | 0.9 | 1.5 | 0.5 | |
| 8 | 2.6 | 1.1 | 1.5 | 0.9 | |
| CP * | 2.9 | 1.1 | 1.4 | 0.4 | |
| Control | - | 0.3 | 0.1 | 0.2 | 0.1 |
| LNCaP | 4 | 38.4 | 3.3 | 1.9 | 0.8 |
| 5 | 47.7 | 4.7 | 8.8 | 1.8 | |
| 8 | 45.3 | 3.9 | 7.9 | 2.2 | |
| CP | 26.8 | 3.9 | 0.7 | 1.1 | |
| Control | - | 0.6 | 0.1 | 0.5 | 0.2 |
| DU145 | 4 | 23.5 | 3.5 | 24.7 | 3.5 |
| 5 | 23.9 | 2.6 | 30.5 | 3.6 | |
| 8 | 24.1 | 2.1 | 29.8 | 4.9 | |
| CP | 20.8 | 3.5 | 0.3 | 0.2 |
| Compound | LnCaP | DU145 | PC3 | |
|---|---|---|---|---|
| MTT test | 2 | + | + + | + + + |
| 4 | + + | + + | + + + | |
| 5 | + + | + + + | + + + | |
| 8 | + + | + + + | + + | |
| LDH test | 2 | + | + | + + + |
| 4 | + + | + + | + + + | |
| 5 | + + + | + + + | + + + | |
| 8 | + + + | + + + | + + + | |
| Apoptosis assay | 2 | + | + | + + + |
| 4 | + + | + + | + + + | |
| 5 | + + | + + | + + + | |
| 8 | + + | + ++ | + + + | |
| IL-6 release inhibition | 2 | + | + | + + + |
| 4 | + + | + + | + + + | |
| 5 | + + | + + + | + + + | |
| 8 | + + | + + + | + |
| Accession | Name of Enzyme | PC3 | RWPE-1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 2 | 4 | 5 | 8 | CP | Control | 2 | 4 | 5 | 8 | CP | ||
| Lipid transport and signaling | |||||||||||||
| FABP5_ HUMAN | Fatty acid-binding protein 5 | 100 | 26.2 | 60.8 | 88.9 | 100.8 | 92.4 | - | - | - | - | - | - |
| AT8A1_ HUMAN | Phospholipid-transporting ATPase IA | 100 | 142.2 | 247 | 313.9 | 249.5 | 124.5 | 100 | 111.2 | 109.3 | 114.5 | 107.6 | 121.2 |
| NPC2_ HUMAN | NPC intracellular cholesterol transporter 2 | 100 | 184.2 | 93.3 | 69.5 | 131.7 | 91.3 | 100 | 92.7 | 98.2 | 94.7 | 94.5 | 91.2 |
| CAV1_ HUMAN | Caveolin-1 | 100 | 94.3 | 86.2 | 59.1 | 113.8 | 89.2 | 100 | 94.8 | 87.1 | 85.3 | 82.7 | 92.5 |
| Lipid anabolism and storage | |||||||||||||
| FAS_HUMAN | Fatty acid synthase | 100 | 101.1 | 48.1 | 24.1 | 62.4 | 87.4 | 100 | 122.1 | 113.6 | 118.6 | 106.6 | 94.5 |
| ACACB_ HUMAN | Acetyl-CoA carboxylase 2 | 100 | 88.2 | 21.4 | 22.8 | 56.5 | 89.3 | 100 | 89.4 | 96.4 | 93.0 | 83.9 | 89.3 |
| PLIN3_ HUMAN | Perilipin-3 | 100 | 75.2 | 81.1 | 42.7 | 91.2 | 86.3 | 100 | 105.2 | 113.4 | 116.2 | 132.3 | 95.2 |
| Lipid catabolism | |||||||||||||
| DECR_ HUMAN | 2,4-dienoyl-CoA reductase, mitochondrial | 100 | 35.5 | 51.7 | 64.4 | 84.7 | 82.7 | 100 | 91.3 | 92.5 | 109.2 | 102.4 | 96.3 |
| THIL_ HUMAN | Acetyl-CoA acetyltransferase, mitochondrial | 100 | 54.2 | 71.2 | 51.6 | 119.1 | 83.4 | 100 | 123.4 | 132.3 | 129.3 | 108.3 | 94.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrzanowska, A.; Olejarz, W.; Kubiak-Tomaszewska, G.; Ciechanowicz, A.K.; Struga, M. The Effect of Fatty Acids on Ciprofloxacin Cytotoxic Activity in Prostate Cancer Cell Lines—Does Lipid Component Enhance Anticancer Ciprofloxacin Potential? Cancers 2022, 14, 409. https://doi.org/10.3390/cancers14020409
Chrzanowska A, Olejarz W, Kubiak-Tomaszewska G, Ciechanowicz AK, Struga M. The Effect of Fatty Acids on Ciprofloxacin Cytotoxic Activity in Prostate Cancer Cell Lines—Does Lipid Component Enhance Anticancer Ciprofloxacin Potential? Cancers. 2022; 14(2):409. https://doi.org/10.3390/cancers14020409
Chicago/Turabian StyleChrzanowska, Alicja, Wioletta Olejarz, Grażyna Kubiak-Tomaszewska, Andrzej K. Ciechanowicz, and Marta Struga. 2022. "The Effect of Fatty Acids on Ciprofloxacin Cytotoxic Activity in Prostate Cancer Cell Lines—Does Lipid Component Enhance Anticancer Ciprofloxacin Potential?" Cancers 14, no. 2: 409. https://doi.org/10.3390/cancers14020409
APA StyleChrzanowska, A., Olejarz, W., Kubiak-Tomaszewska, G., Ciechanowicz, A. K., & Struga, M. (2022). The Effect of Fatty Acids on Ciprofloxacin Cytotoxic Activity in Prostate Cancer Cell Lines—Does Lipid Component Enhance Anticancer Ciprofloxacin Potential? Cancers, 14(2), 409. https://doi.org/10.3390/cancers14020409