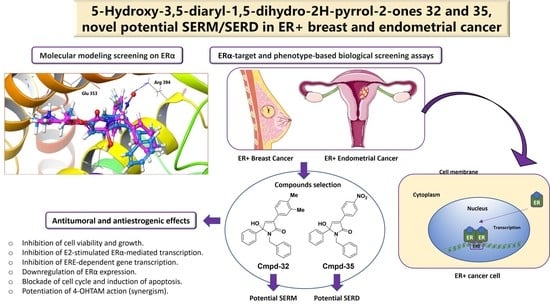
Discovery of Highly Functionalized 5-hydroxy-2H-pyrrol-2-ones That Exhibit Antiestrogenic Effects in Breast and Endometrial Cancer Cells and Potentiate the Antitumoral Effect of Tamoxifen
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. General Procedure for the Synthesis of 5-hydroxy-3,5-diaryl-1,5-dihydro-2H-pyrrol-2-ones (4–50)
2.2.1. 1-cyclohexyl-5-hydroxy-3,5-diphenyl-1H-pyrrol-2(5H)-one (4)
2.2.2. 1-benzyl-5-hydroxy-3,5-diphenyl-1H-pyrrol-2(5H)-one (5)
2.2.3. 5-hydroxy-1-(4-methoxyphenyl)-3,5-diphenyl-1H-pyrrol-2(5H)-one (6)
2.2.4. 1-cyclohexyl-5-hydroxy-5-(4-methoxyphenyl)-3-phenyl-1H-pyrrol-2(5H)-one (7)
2.2.5. 1-benzyl-5-hydroxy-5-(4-methoxyphenyl)-3-phenyl-1H-pyrrol-2(5H)-one (8)
2.2.6. 5-hydroxy-1,5-bis(4-methoxyphenyl)-3-phenyl-1H-pyrrol-2(5H)-one (9)
2.2.7. 1-cyclohexyl-3-(3-fluorophenyl)-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (10)
2.2.8. 1-benzyl-3-(3-fluorophenyl)-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (11)
2.2.9. 3-(3-fluorophenyl)-5-hydroxy-1-(4-methoxyphenyl)-5-phenyl-1H-pyrrol-2(5H)-one (12)
2.2.10. 1-cyclohexyl-3-(4-fluorophenyl)-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (13)
2.2.11. 1-benzyl-3-(4-fluorophenyl)-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (14)
2.2.12. 3-(4-fluorophenyl)-5-hydroxy-1-(4-methoxyphenyl)-5-phenyl-1H-pyrrol-2(5H)-one (15)
2.2.13. 1-cyclohexyl-3-(3-fluoro-4-methoxyphenyl)-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (16)
2.2.14. 1-benzyl-3-(3-fluoro-4-methoxyphenyl)-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (17)
2.2.15. 3-(3-fluoro-4-methoxyphenyl)-5-hydroxy-1-(4-methoxyphenyl)-5-phenyl-1H-pyrrol-2(5H)-one (18)
2.2.16. 3-(4-chlorophenyl)-1-cyclohexyl-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (19)
2.2.17. 1-benzyl-3-(4-chlorophenyl)-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (20)
2.2.18. 3-(4-chlorophenyl)-5-hydroxy-1-(4-methoxyphenyl)-5-phenyl-1H-pyrrol-2(5H)-one (21)
2.2.19. 3-(4-bromophenyl)-1-cyclohexyl-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (22)
2.2.20. 1-benzyl-3-(4-bromophenyl)-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (23)
2.2.21. 3-(4-bromophenyl)-5-hydroxy-1-(4-methoxyphenyl)-5-phenyl-1H-pyrrol-2(5H)-one (24)
2.2.22. 3-(benzo[d][1,3]dioxol-5-yl)-1-cyclohexyl-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (25)
2.2.23. 3-(benzo[d][1,3]dioxol-5-yl)-1-benzyl-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (26)
2.2.24. 3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-1-(4-methoxyphenyl)-5-phenyl-1H-pyrrol-2(5H)-one (27)
2.2.25. 1-cyclohexyl-5-hydroxy-3-(4-methoxyphenyl)-5-phenyl-1H-pyrrol-2(5H)-one (28)
2.2.26. 1-benzyl-5-hydroxy-3-(4-methoxyphenyl)-5-phenyl-1H-pyrrol-2(5H)-one (29)
2.2.27. 5-hydroxy-1,3-bis(4-methoxyphenyl)-5-phenyl-1H-pyrrol-2(5H)-one (30)
2.2.28. 1-cyclohexyl-3-(3,4-dimethylphenyl)-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (31)
2.2.29. 1-benzyl-3-(3,4-dimethylphenyl)-5-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one (32)
2.2.30. 3-(3,4-dimethylphenyl)-5-hydroxy-1-(4-methoxyphenyl)-5-phenyl-1H-pyrrol-2(5H)-one (33)
2.2.31. 1-cyclohexyl-5-hydroxy-3-(4-nitrophenyl)-5-phenyl-1H-pyrrol-2(5H)-one (34)
2.2.32. 1-benzyl-5-hydroxy-3-(4-nitrophenyl)-5-phenyl-1H-pyrrol-2(5H)-one (35)
2.2.33. 1-cyclohexyl-5-hydroxy-5-(3-methoxyphenyl)-3-(3-nitrophenyl)-1H-pyrrol-2(5H)-one (36)
2.2.34. 1-benzyl-5-hydroxy-5-(3-methoxyphenyl)-3-(3-nitrophenyl)-1H-pyrrol-2(5H)-one (37)
2.2.35. 5-hydroxy-5-(3-methoxyphenyl)-1-(4-methoxyphenyl)-3-(3-nitrophenyl)-1H-pyrrol-2(5H)-one (38)
2.2.36. 1-cyclohexyl-5-hydroxy-3-(4-methoxyphenyl)-5-(3-nitrophenyl)-1H-pyrrol-2(5H)-one (39)
2.2.37. 1-benzyl-5-hydroxy-3-(4-methoxyphenyl)-5-(3-nitrophenyl)-1H-pyrrol-2(5H)-one (40)
2.2.38. 5-hydroxy-1,3-bis(4-methoxyphenyl)-5-(3-nitrophenyl)-1H-pyrrol-2(5H)-one (41)
2.2.39. 1-cyclohexyl-5-hydroxy-3-(4-methoxyphenyl)-5-(4-nitrophenyl)-1H-pyrrol-2(5H)-one (42)
2.2.40. 1-benzyl-5-hydroxy-3-(4-methoxyphenyl)-5-(4-nitrophenyl)-1H-pyrrol-2(5H)-one (43)
2.2.41. 5-hydroxy-1,3-bis(4-methoxyphenyl)-5-(4-nitrophenyl)-1H-pyrrol-2(5H)-one (44)
2.2.42. 1-cyclohexyl-5-hydroxy-3-(3-methoxyphenyl)-5-(4-nitrophenyl)-1H-pyrrol-2(5H)-one (45)
2.2.43. 1-benzyl-5-hydroxy-3-(3-methoxyphenyl)-5-(4-nitrophenyl)-1H-pyrrol-2(5H)-one (46)
2.2.44. 5-hydroxy-3-(3-methoxyphenyl)-1-(4-methoxyphenyl)-5-(4-nitrophenyl)-1H-pyrrol-2(5H)-one (47)
2.2.45. 1-cyclohexyl-5-hydroxy-3-(2-methoxyphenyl)-5-(4-nitrophenyl)-1H-pyrrol-2(5H)-one (48)
2.2.46. 1-benzyl-5-hydroxy-3-(2-methoxyphenyl)-5-(4-nitrophenyl)-1H-pyrrol-2(5H)-one (49)
2.2.47. 5-hydroxy-3-(2-methoxyphenyl)-1-(4-methoxyphenyl)-5-(4-nitrophenyl)-1H-pyrrol-2(5H)-one (50)
2.3. Computational Studies
2.3.1. Protein Preparation and Docking Studies
2.3.2. In Silico ADME and Drug-Likeness Analyses
2.4. Biological Evaluation
2.4.1. Reagents
2.4.2. Cells
2.4.3. Transcriptional Activity Assays
2.4.4. Cell Viability Assays
2.4.5. Human ERα Competitor Binding Assay
2.4.6. Rat ER Competitor Binding Assay
2.4.7. Real-Time Monitoring of 2D-3D Tumor Growth
2.4.8. Alkaline Phosphatase Assay
2.4.9. Cell Cycle and Apoptosis Analysis
2.4.10. Immunoblotting
2.4.11. Immunofluorescence
2.4.12. Quantitative Real-Time PCR
2.4.13. Drug Combination Assays
2.4.14. Statistical Analysis
3. Results and Discussion
3.1. Virtual Screening and Synthesis of Highly Functionalized 5-hydroxy-2H-pyrrol-2-ones as ER Modulators
3.2. 5-hydroxy-2H-pyrrol-2-ones Inhibit Cell Viability of ER+ Breast Cancer Cells
3.3. 5-hydroxy-2H-pyrrol-2-ones Modulate ER-Dependent Transcription in Breast Cancer Cells
3.4. 5-hydroxy-2H-pyrrol-2-ones 32 and 35 Bind to Human and Rat ERα
3.5. 5-hydroxy-2H-pyrrol-2-ones 32 and 35 Inhibit Cell Growth of ER+ Breast Cancer Cells
3.6. 5-hydroxy-2H-pyrrol-2-ones 32 and 35 Inhibit E2-Dependent Growth of Breast Cancer Cells
3.7. 5-hydroxy-2H-pyrrol-2-ones 32 and 35 Inhibit E2-Dependent Induction of ERα-Regulated Genes in ER+ Breast and Endometrial Cancer Cells
3.8. 5-hydroxy-2H-pyrrol-2-ones 32 and 35 Block Cell Cycle Entry and Induce Apoptosis in ER+ BC Cells
3.9. 5-hydroxy-2H-pyrrol-2-ones 32 and 35 Decrease ERα Protein Level
3.10. 5-hydroxy-2H-pyrrol-2-ones 32 and 35 Potentiate Antitumoral Effect of 4-OHTAM on ER+ Breast Cancer Cells
3.11. In Silico ADME Predictions of 5-hydroxy-2H-pyrrol-2-ones
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Richman, J.; Dowsett, M. Beyond 5 Years: Enduring Risk of Recurrence in Oestrogen Receptor-Positive Breast Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Thomas, C.; Gustafsson, J.Å. The Different Roles of ER Subtypes in Cancer Biology and Therapy. Nat. Rev. Cancer 2011, 11, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen Receptors: How Do They Signal and What Are Their Targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacGregor, J.I.; Jordan, V.C. Basic Guide to the Mechanisms of Antiestrogen Action. Pharmacol. Rev. 1998, 50, 151–196. [Google Scholar] [PubMed]
- Nilsson, S.; Koehler, K.F.; Gustafsson, J.Å. Development of Subtype-Selective Oestrogen Receptor-Based Therapeutics. Nat. Rev. Drug Discov. 2011, 10, 778–792. [Google Scholar] [CrossRef]
- Liang, J.; Shang, Y. Estrogen and Cancer. Annu. Rev. Physiol. 2013, 75, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Johnston, S.R.D.; Dowsett, M. Aromatase Inhibitors for Breast Cancer: Lessons from the Laboratory. Nat. Rev. Cancer 2003, 3, 821–831. [Google Scholar] [CrossRef]
- Ma, C.X.; Reinert, T.; Chmielewska, I.; Ellis, M.J. Mechanisms of Aromatase Inhibitor Resistance. Nat. Rev. Cancer 2015, 15, 261–275. [Google Scholar] [CrossRef]
- Robertson, J.F. Faslodex (ICI 182, 780), a Novel Estrogen Receptor Downregulator--Future Possibilities in Breast Cancer. J. Steroid Biochem. Mol. Biol. 2001, 79, 209–212. [Google Scholar] [CrossRef]
- Xiong, R.; Zhao, J.; Gutgesell, L.M.; Wang, Y.; Lee, S.; Karumudi, B.; Zhao, H.; Lu, Y.; Tonetti, D.A.; Thatcher, G.R.J. Novel Selective Estrogen Receptor Downregulators (SERDs) Developed against Treatment-Resistant Breast Cancer. J. Med. Chem. 2017, 60, 1325–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, V.C. Tamoxifen: Toxicities and Drug Resistance during the Treatment and Prevention of Breast Cancer. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Katzenellenbogen, J.A.; Mayne, C.G.; Katzenellenbogen, B.S.; Greene, G.L.; Chandarlapaty, S. Structural Underpinnings of Oestrogen Receptor Mutations in Endocrine Therapy Resistance. Nat. Rev. Cancer 2018, 18, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Stebbing, J.; Giamas, G.; Murphy, J. Endocrine Resistance in Hormone Receptor Positive Breast Cancer–from Mechanism to Therapy. Front. Endocrinol. 2019, 10, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.K.; Bihani, T. Selective Estrogen Receptor Modulators (SERMs) and Selective Estrogen Receptor Degraders (SERDs) in Cancer Treatment. Pharmacol. Ther. 2018, 186, 1–24. [Google Scholar] [CrossRef]
- Shang, Y. Molecular Mechanisms of Oestrogen and SERMs in Endometrial Carcinogenesis. Nat. Rev. Cancer 2006, 6, 360–368. [Google Scholar] [CrossRef]
- Johnson, S.M.; Maleki-Dizaji, M.; Styles, J.A.; White, I.N.H. Ishikawa Cells Exhibit Differential Gene Expression Profiles in Response to Oestradiol or 4-Hydroxytamoxifen. Endocr. Relat. Cancer 2007, 14, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Weiss, D.J.; Gurpide, E. Non-Genomic Effects of Estrogens and Antiestrogens. J. Steroid Biochem. 1988, 31, 671–676. [Google Scholar] [CrossRef]
- Díaz, M. Triphenylethylene Antiestrogen-Induced Acute Relaxation of Mouse Duodenal Muscle. Possible Involvement of Ca2+ Channels. Eur. J. Pharmacol. 2002, 445, 257–266. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wang, H.; Wang, Y.; He, Q.; Xia, X.; Hu, Z.-Y.; Ouyang, Q. Clinical and Genetic Risk Factors for Fulvestrant Treatment in Post-Menopause ER-Positive Advanced Breast Cancer Patients. J. Transl. Med. 2019, 17, 27. [Google Scholar] [CrossRef]
- Huang, D.; Yang, F.; Wang, Y.; Guan, X. Mechanisms of Resistance to Selective Estrogen Receptor Down-Regulator in Metastatic Breast Cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Giessrigl, B.; Schmidt, W.M.; Kalipciyan, M.; Jeitler, M.; Bilban, M.; Gollinger, M.; Krieger, S.; Jäger, W.; Mader, R.M.; Krupitza, G. Fulvestrant Induces Resistance by Modulating GPER and CDK6 Expression: Implication of Methyltransferases, Deacetylases and the HSWI/SNF Chromatin Remodelling Complex. Br. J. Cancer 2013, 109, 2751–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, S.; Bender, S.G.; Yahn, R.; Till, N.A.; Varady, S.; LaLonde, R.L. Searching for an Ideal SERM: Mining Tamoxifen Structure-Activity Relationships. Bioorg. Med. Chem. Lett. 2021, 52, 128383. [Google Scholar] [CrossRef] [PubMed]
- Mowery, P.; Banales Mejia, F.; Franceschi, C.L.; Kean, M.H.; Kwansare, D.O.; Lafferty, M.M.; Neerukonda, N.D.; Rolph, C.E.; Truax, N.J.; Pelkey, E.T. Synthesis and Evaluation of the Anti-Proliferative Activity of Diaryl-3-Pyrrolin-2-Ones and Fused Analogs. Bioorg. Med. Chem. Lett. 2017, 27, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Joksimović, N.; Petronijević, J.; Janković, N.; Baskić, D.; Popović, S.; Todorović, D.; Matić, S.; Bogdanović, G.A.; Vraneš, M.; Tot, A.; et al. Synthesis, Characterization, Anticancer Evaluation and Mechanisms of Cytotoxic Activity of Novel 3-Hydroxy-3-Pyrrolin-2-Ones Bearing Thenoyl Fragment: DNA, BSA Interactions and Molecular Docking Study. Bioorg. Chem. 2019, 88, 102954. [Google Scholar] [CrossRef]
- Li Petri, G.; Raimondi, M.V.; Spanò, V.; Holl, R.; Barraja, P.; Montalbano, A. Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds. Top. Curr. Chem. 2021, 379, 34. [Google Scholar] [CrossRef]
- Joksimović, N.; Petronijević, J.; Milović, E.; Janković, N.; Baskić, D.; Popović, S.; Todorović, D.; Matić, S.; Vraneš, M.; Tot, A. Synthesis, Characterization, Antitumor Potential, BSA and DNA Binding Properties, and Molecular Docking Study of Some Novel 3-Hydroxy-3- Pyrrolin-2-Ones. Med. Chem. 2022, 18, 337–352. [Google Scholar] [CrossRef]
- Miyazaki, H.; Miyake, T.; Terakawa, Y.; Ohmizu, H.; Ogiku, T.; Ohtani, A. Evaluation of Pyrrolin-2-One Derivatives Synthesized by a New Practical Method as Inhibitors of Plasminogen Activator Inhibitor-1 (PAI-1). Bioorg. Med. Chem. Lett. 2010, 20, 546–548. [Google Scholar] [CrossRef]
- Bosch, J.; Roca, T.; Catena, J.L.; Llorens, O.; Pérez, J.J.; Lagunas, C.; Fernández, A.G.; Miquel, I.; Fernández-Serrat, A.; Farrerons, C. Synthesis and Biological Evaluation of 1,3,4-Triaryl-3-Pyrrolin-2-Ones, a New Class of Selective Cyclooxygenase-2 Inhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 1745–1748. [Google Scholar] [CrossRef]
- Eldridge, M.D.; Murray, C.W.; Auton, T.R.; Paolini, G.V.; Mee, R.P. Empirical Scoring Functions: I. The Development of a Fast Empirical Scoring Function to Estimate the Binding Affinity of Ligands in Receptor Complexes. J. Comput. Aided. Mol. Des. 1997, 11, 425–445. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Wilson, V.S.; Bobseine, K.; Gray, L.E. Development and Characterization of a Cell Line That Stably Expresses an Estrogen-Responsive Luciferase Reporter for the Detection of Estrogen Receptor Agonist and Antagonists. Toxicol. Sci. 2004, 81, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, V.S.; Bobseine, K.; Lambright, C.R.; Gray, L.E. A Novel Cell Line, MDA-Kb2, That Stably Expresses an Androgen- and Glucocorticoid-Responsive Reporter for the Detection of Hormone Receptor Agonists and Antagonists. Toxicol. Sci. 2002, 66, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assay. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Marrero-Alonso, J.; Morales, A.; García Marrero, B.; Boto, A.; Marín, R.; Cury, D.; Gómez, T.; Fernández-Pérez, L.; Lahoz, F.; Díaz, M. Unique SERM-like Properties of the Novel Fluorescent Tamoxifen Derivative FLTX1. Eur. J. Pharm. Biopharm. 2013, 85, 898–910. [Google Scholar] [CrossRef]
- Holinka, C.F.; Hata, H.; Kuramoto, H.; Gurpide, E. Responses to Estradiol in a Human Endometrial Adenocarcinoma Cell Line (Ishikawa). J. Steroid Biochem. 1986, 24, 85–89. [Google Scholar] [CrossRef]
- Faunes, F.; Hayward, P.; Descalzo, S.M.; Chatterjee, S.S.; Balayo, T.; Trott, J.; Christoforou, A.; Ferrer-Vaquer, A.; Hadjantonakis, A.-K.; Dasgupta, R.; et al. A Membrane-Associated β-Catenin/Oct4 Complex Correlates with Ground-State Pluripotency in Mouse Embryonic Stem Cells. Development 2013, 140, 1171–1183. [Google Scholar] [CrossRef] [Green Version]
- Lou, X.; Kang, M.; Xenopoulos, P.; Muñoz-Descalzo, S.; Hadjantonakis, A.-K. A Rapid and Efficient 2D/3D Nuclear Segmentation Method for Analysis of Early Mouse Embryo and Stem Cell Image Data. Stem Cell Rep. 2014, 2, 382–397. [Google Scholar] [CrossRef]
- Verde, G.; De Llobet, L.I.; Wright, R.H.G.; Quilez, J.; Peiró, S.; Le Dily, F.; Beato, M. Unliganded Progesterone Receptor Governs Estrogen Receptor Gene Expression by Regulating DNA Methylation in Breast Cancer Cells. Cancers 2018, 10, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angelis, M.; Stossi, F.; Carlson, K.A.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A. Indazole Estrogens: Highly Selective Ligands for the Estrogen Receptor Beta. J. Med. Chem. 2005, 48, 1132–1144. [Google Scholar] [CrossRef]
- Makar, S.; Saha, T.; Swetha, R.; Gutti, G.; Kumar, A.; Singh, S.K. Rational Approaches of Drug Design for the Development of Selective Estrogen Receptor Modulators (SERMs), Implicated in Breast Cancer. Bioorg. Chem. 2020, 94, 103380. [Google Scholar] [CrossRef]
- Adib, M.; Mahdavi, M.; Noghani, M.A.; Bijanzadeh, H.R. Reaction between Isocyanides and Chalcones: An Efficient Solvent-Free Synthesis of 5-Hydroxy-3,5-Diaryl-1,5-Dihydro-2H-Pyrrol-2-Ones. Tetrahedron Lett. 2007, 48, 8056–8059. [Google Scholar] [CrossRef]
- Yadav, Y.; MacLean, E.D.; Bhattacharyya, A.; Parmar, V.S.; Balzarini, J.; Barden, C.J.; Too, C.K.L.; Jha, A. Design, Synthesis and Bioevaluation of Novel Candidate Selective Estrogen Receptor Modulators. Eur. J. Med. Chem. 2011, 46, 3858–3866. [Google Scholar] [CrossRef]
- Furth, P.A. STAT Signaling in Different Breast Cancer Sub-Types. Mol. Cell. Endocrinol. 2014, 382, 612–615. [Google Scholar] [CrossRef] [Green Version]
- Lewis-Wambi, J.S.; Kim, H.; Curpan, R.; Grigg, R.; Sarker, M.A.; Jordan, V.C. The Selective Estrogen Receptor Modulator Bazedoxifene Inhibits Hormone-Independent Breast Cancer Cell Growth and down-Regulates Estrogen Receptor α and Cyclin D1. Mol. Pharmacol. 2011, 80, 610–620. [Google Scholar] [CrossRef] [Green Version]
- Kulkoyluoglu-Cotul, E.; Arca, A.; Madak-Erdogan, Z. Crosstalk between Estrogen Signaling and Breast Cancer Metabolism. Trends Endocrinol. Metab. 2019, 30, 25–38. [Google Scholar] [CrossRef]
- Siersbæk, R.; Scabia, V.; Nagarajan, S.; Chernukhin, I.; Papachristou, E.K.; Broome, R.; Johnston, S.J.; Joosten, S.E.P.; Green, A.R.; Kumar, S.; et al. IL6/STAT3 Signaling Hijacks Estrogen Receptor α Enhancers to Drive Breast Cancer Metastasis. Cancer Cell 2020, 38, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A.; Pompliano, D.L.; Meek, T.D. Drug-Target Residence Time and Its Implications for Lead Optimization. Nat. Rev. Drug Discov. 2006, 5, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, R.; Ward, R.A. Structure-Based Design of Targeted Covalent Inhibitors. Chem. Soc. Rev. 2018, 47, 3816–3830. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-Based Drug Screen: Considerations and Practical Approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Redmond, C.K.; Kavanah, M.; Cronin, W.M.; Vogel, V.; Robidoux, A.; Dimitrov, N.; Atkins, J.; et al. Tamoxifen for Prevention of Breast Cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 1998, 90, 1371–1388. [Google Scholar] [CrossRef] [Green Version]
- Bhat, K.P.L.; Pezzuto, J.M. Resveratrol Exhibits Cytostatic and Antiestrogenic Properties with Human Endometrial Adenocarcinoma (Ishikawa) Cells. Cancer Res. 2001, 61, 6137–6144. [Google Scholar]
- Wober, J.; Weißwange, I.; Vollmer, G. Stimulation of Alkaline Phosphatase Activity in Ishikawa Cells Induced by Various Phytoestrogens and Synthetic Estrogens. Proc. J. Steroid Biochem. Mol. Biol. 2002, 83, 227–233. [Google Scholar] [CrossRef]
- Burris, T.P.; Solt, L.A.; Wang, Y.; Crumbley, C.; Banerjee, S.; Griffett, K.; Lundasen, T.; Hughes, T.; Kojetin, D.J. Nuclear Receptors and Their Selective Pharmacologic Modulators. Pharmacol. Rev. 2013, 65, 710–778. [Google Scholar] [CrossRef]
- Hu, R.; Hilakivi-Clarke, L.; Clarke, R. Molecular Mechanisms of Tamoxifen-Associated Endometrial Cancer (Review). Oncol. Lett. 2015, 9, 1495–1501. [Google Scholar] [CrossRef] [Green Version]
- Kajstura, M.; Halicka, H.D.; Pryjma, J.; Darzynkiewicz, Z. Discontinuous Fragmentation of Nuclear DNA during Apoptosis Revealed by Discrete “Sub-G1” Peaks on DNA Content Histograms. Cytom. Part A J. Int. Soc. Anal. Cytol. 2007, 71, 125–131. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer Review Douglas. Cell 2000, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Deroo, B.J.; Korach, K.S. Estrogen Receptors and Human Disease. J. Clin. Invest. 2006, 116, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, M.G. Action of “Pure” Antiestrogens in Inhibiting Estrogen Receptor Action. Breast Cancer Res. Treat. 1993, 26, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhou, W.; Hafner, M.; Blake, R.A.; Chalouni, C.; Chen, I.P.; De Bruyn, T.; Giltnane, J.M.; Hartman, S.J.; Heidersbach, A.; et al. Therapeutic Ligands Antagonize Estrogen Receptor Function by Impairing Its Mobility. Cell 2019, 178, 949–963.e18. [Google Scholar] [CrossRef]
- Smith, C.L.; O’Malley, B.W. Coregulator Function: A Key to Understanding Tissue Specificity of Selective Receptor Modulators. Endocr. Rev. 2004, 25, 45–71. [Google Scholar] [CrossRef]
- Kisselev, A.F.; Goldberg, A.L. Proteasome Inhibitors: From Research Tools to Drug Candidates. Chem. Biol. 2001, 8, 739–758. [Google Scholar] [CrossRef] [Green Version]
- Aranda-Tavío, H.; Recio, C.; Martín-Acosta, P.; Guerra-Rodríguez, M.; Brito-Casillas, Y.; Blanco, R.; Junco, V.; León, J.; Montero, J.C.; Gandullo-Sánchez, L.; et al. JKST6, a Novel Multikinase Modulator of the BCR-ABL1/STAT5 Signaling Pathway That Potentiates Direct BCR-ABL1 Inhibition and Overcomes Imatinib Resistance in Chronic Myelogenous Leukemia. Biomed. Pharmacother. 2021, 144, 112330. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
















| ER+ Cells | ER− Cells | Non-Malignant Cells | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCF-7 | MCF-7/BUS | T47D | ISHIKAWA | SK-BR-3 | BT-549 | Hs-578T | MDA-MB-231 | MCF-10A | VERO | PBMC | |
| ID Compound | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| DOXO | 0.07 ± 0.01 | 0.05 ± 0.02 | 0.15 ± 0.02 | 0.09 ± 0.03 | 0.04 ± 0.01 | 0.19 ± 0.04 | 0.37 ± 0.05 | 0.25 ± 0.07 | 0.09 ± 0.01 | 2.62 ± 0.24 | 1.47 ± 0.69 |
| 4-OHTAM | 4.43 ± 1.03 | 0.09 ± nd | 5.9 ± 0.76 | 6.16 ± 0.8 | 7.85 ± 1.95 | >10 ± 0 | >10 ± 0 | >10 ± 0 | 5.3 ± 0.28 | 10.79 ± 0.13 | nd ± nd |
| ICI | 0.52 ± 0.16 | 0.0006 ± 0 | 14.91 ± 5.19 | >10 ± 0 | >50 ± 0 | >25 ± 0 | >25 ± 0 | >25 ± 0 | >10 ± 0 | >10 ± 0 | nd ± nd |
| 4 | >10 ± nd | nd ± nd | >10 ± 0 | >10 ± 0 | >10 ± nd | nd ± nd | >10 ± nd | >10 ± nd | 7.17 ± 2.07 | >25 ± 0 | nd ± nd |
| 9 | >10 ± nd | >10 ± 0 | >10 ± 0 | nd ± nd | >10 ± nd | >10 ± nd | >10 ± nd | >10 ± 0 | nd ± nd | nd ± nd | nd ± nd |
| 16 | >10 ± nd | >10 ± 0 | >10 ± 0 | >10 ± 0 | >10 ± nd | >10 ± nd | >10 ± nd | >10 ± nd | nd ± nd | nd ± nd | nd ± nd |
| 26 | >10 ± nd | nd ± nd | >10 ± 0 | 10.11 ± 0.11 | >10 ± nd | nd ± nd | >10 ± nd | 9.71 ± 0.3 | nd ± nd | nd ± nd | nd ± nd |
| 32 | 2.54 ± 0.49 | 5.32 ± 2.09 | 10.64 ± 0.58 | 0.27 ± 0.09 | 1.51 ± 0.09 | 4.89 ± 0.96 | >10 ± 0 | 7.63 ± 2.38 | 0.08 ± 0 | 14.74 ± 1.31 | >30 ± 0 |
| 35 | 2.59 ± 0.34 | 2.64 ± 0.88 | 5.31 ± 0.47 | 1.29 ± 0.38 | 2 ± 0.18 | 4.19 ± 0 | 8.22 ± 1.78 | 9.08 ± 0.93 | 3.16 ± 0.09 | 9.61 ± 0.16 | 17.26 ± 6.48 |
| 38 | >10 ± nd | nd ± nd | >10 ± 0 | >10 ± 0 | >10 ± nd | nd ± nd | >10 ± nd | >10 ± nd | >10 ± 0 | >25 ± 0 | nd ± nd |
| 43 | 6.54 ± 0.41 | 2.85 ± 1.24 | 8.57 ± 0.43 | 3.47 ± 0.94 | 2.16 ± 0.05 | 6.23 ± 3.48 | >10 ± nd | >10 ± 0 | nd ± nd | nd ± nd | nd ± nd |
| 49 | 3.47 ± 1.12 | 5 ± 1.79 | >10 ± 0 | 4.82 ± 1.84 | >10 ± nd | >10 ± nd | >10 ± nd | 7.94 ± 2.07 | 0.31 ± 0.03 | nd ± nd | nd ± nd |
| 50 | 6.17 ± 2.66 | nd ± nd | nd ± nd | nd ± nd | >10 ± nd | nd ± nd | nd ± nd | nd ± nd | nd ± nd | nd ± nd | nd ± nd |
| A | B | ||
|---|---|---|---|
| VEH | E2 | ||
| ID Compound | E/Emax (%) | E/Emax (%) | IC50 (µM) |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| E2 | 99.98 ± 11.29 | nd | nd |
| 4-OHTAM | −7.14 ± 3.57 | −10.62 ± 0.64 | <0.03 ± 0 |
| ICI | −9.15 ± 3.99 | −9.89 ± 2.33 | 1.90 × 10−4 ± 3.00 × 10−5 |
| 4 | 0.01 ± 1.02 | 39.73 ± 14.82 | 3.4 ± 1.64 |
| 5 | 3.33 ± 1.32 | 111.82 ± 25.16 | >10 ± 0 |
| 8 | 0.58 ± 1.79 | 68.75 ± 13.82 | >10 ± 0 |
| 10 | 4.02 ± 2.12 | 92.97 ± 14.37 | >10 ± 0 |
| 16 | 16.37 ± 7.86 | 83.04 ± 7.49 | >10 ± 0 |
| 20 | −0.29 ± 1.67 | 77.75 ± 27.24 | >10 ± 0 |
| 21 | −1.43 ± 1.53 | 119.51 ± 23.29 | >10 ± 0 |
| 23 | 1.32 ± 3.15 | 77.59 ± 28.28 | >10 ± 0 |
| 25 | 3.89 ± 3.73 | 70.34 ± 11.06 | >10 ± 0 |
| 26 | 0.11 ± 2.78 | 49.7 ± 15.05 | 8.35 ± 1.35 |
| 32 | −0.32 ± 0.73 | 105.29 ± 18.23 | >10 ± 0 |
| 35 | −11.82 ± 5.37 | 29.72 ± 9.92 | 8.2 ± 1.19 |
| 37 | −0.67 ± 0.92 | 126.11 ± 21.36 | >10 ± 0 |
| 38 | −0.87 ± 3.05 | 61.65 ± 5.52 | 3.58 ± 2.4 |
| 39 | 1.94 ± 0.88 | 101.35 ± 0.27 | >10 ± 0 |
| 40 | −3.22 ± 1.32 | 31.89 ± 11.43 | 8.77 ± 0.59 |
| 43 | −1.01 ± 2.56 | 172.56 ± 28.04 | >10 ± 0 |
| 46 | −6.02 ± 3.4 | 54.1 ± 13.33 | 6.54 ± 3.47 |
| 49 | 3.08 ± 4.84 | 97.95 ± 13.32 | >10 ± 0 |
| 50 | −0.16 ± 0.45 | 33.68 ± 11.2 | nd ± nd |
| ID Compound | IC50 (µM) |
|---|---|
| Mean ± SEM | |
| E2 | 1.01 × 10−4 ± 3.16 × 10−10 |
| DES | 8.40 × 10−5 ± 4.00 × 10−11 |
| 4-OHTAM | 7.98 × 10−5 ± 2.95 × 10−12 |
| 4 | 13.3 ± 4.66 × 10−7 |
| 26 | 18.2 ± 4.83 × 10−8 |
| 32 | 32.4 ± 7.67 × 10−9 |
| 35 | 27 ± 7.90 × 10−8 |
| 38 | 26.8 ± 6.38 × 10−7 |
| 49 | 30.6 ± 4.89 × 10−8 |
| AUC (%) | ||
|---|---|---|
| ID Compound | IC50 (μM) | EC50 (μM) |
| Mean ± SEM | Mean ± SEM | |
| E2 | nd ± nd | 7.10 × 10−7 ± 2.60 × 10−7 |
| PPT | nd ± nd | 1.57 × 10−4 ± 6.29 × 10−5 |
| 4-OHTAM | 1.18 × 10−4 ± 0 | null ± null |
| ICI | 2.83 × 10−4 ± 1.12 × 10−4 | null ± null |
| 4 | 4.97 ± 0.35 | null ± null |
| 9 | >10 ± 0 | null ± null |
| 16 | 2.86 ± 2.1 | null ± null |
| 32 | 0.42 ± 0.13 | null ± null |
| 35 | 0.81 ± 0.46 | null ± null |
| 38 | 4.27 ± 0.34 | null ± null |
| 43 | 3.95 ± 0.75 | null ± null |
| 49 | 1.89 ± 0.63 | null ± null |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Rodríguez, M.; López-Rojas, P.; Amesty, Á.; Aranda-Tavío, H.; Brito-Casillas, Y.; Estévez-Braun, A.; Fernández-Pérez, L.; Guerra, B.; Recio, C. Discovery of Highly Functionalized 5-hydroxy-2H-pyrrol-2-ones That Exhibit Antiestrogenic Effects in Breast and Endometrial Cancer Cells and Potentiate the Antitumoral Effect of Tamoxifen. Cancers 2022, 14, 5174. https://doi.org/10.3390/cancers14215174
Guerra-Rodríguez M, López-Rojas P, Amesty Á, Aranda-Tavío H, Brito-Casillas Y, Estévez-Braun A, Fernández-Pérez L, Guerra B, Recio C. Discovery of Highly Functionalized 5-hydroxy-2H-pyrrol-2-ones That Exhibit Antiestrogenic Effects in Breast and Endometrial Cancer Cells and Potentiate the Antitumoral Effect of Tamoxifen. Cancers. 2022; 14(21):5174. https://doi.org/10.3390/cancers14215174
Chicago/Turabian StyleGuerra-Rodríguez, Miguel, Priscila López-Rojas, Ángel Amesty, Haidée Aranda-Tavío, Yeray Brito-Casillas, Ana Estévez-Braun, Leandro Fernández-Pérez, Borja Guerra, and Carlota Recio. 2022. "Discovery of Highly Functionalized 5-hydroxy-2H-pyrrol-2-ones That Exhibit Antiestrogenic Effects in Breast and Endometrial Cancer Cells and Potentiate the Antitumoral Effect of Tamoxifen" Cancers 14, no. 21: 5174. https://doi.org/10.3390/cancers14215174
APA StyleGuerra-Rodríguez, M., López-Rojas, P., Amesty, Á., Aranda-Tavío, H., Brito-Casillas, Y., Estévez-Braun, A., Fernández-Pérez, L., Guerra, B., & Recio, C. (2022). Discovery of Highly Functionalized 5-hydroxy-2H-pyrrol-2-ones That Exhibit Antiestrogenic Effects in Breast and Endometrial Cancer Cells and Potentiate the Antitumoral Effect of Tamoxifen. Cancers, 14(21), 5174. https://doi.org/10.3390/cancers14215174