Clinical Outcomes in COVID-19 Patients Treated with Immunotherapy
Abstract
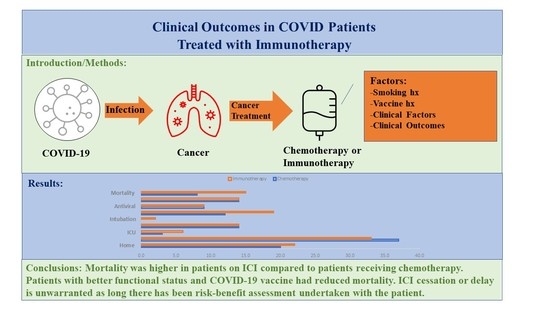
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Clinical Course
3.3. Risk Factors
3.4. Survival Outcomes
4. Discussion
5. Conclusions/Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine transaminase |
| APC | antigen-presenting cells |
| AST | aspartate transaminase |
| COVID-19 | coronavirus disease 2019 |
| CRRT | continuous renal replacement therapy |
| CRP | c-reactive protein |
| CTLA-4 | T-lymphocyte-associated protein 4 |
| ECOG | Eastern Cooperative Oncology Group performance status |
| eGFR | estimated glomerular filtration rate |
| GIST | gastrointestinal stromal tumors |
| ICIs | immune checkpoint inhibitors |
| ICU | intensive care unit |
| IMT | immunotherapy |
| irAE | immune-related adverse event |
| LDH | lactose dehydrogenase |
| MHC | major histocompatibility complex |
| NA | not performed |
| NSCLC | non-small-cell lung cancer |
| NK | natural killer cells |
| OS | overall survival |
| PD-1 | programmed cell death-1 |
| PD-L1 | programmed cell death Ligand 1 |
| RCC | renal cell carcinoma |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| TCR | T-cell receptor |
| WBC | white blood cell |
References
- Fauci, A.S.; Lane, H.C.; Redfield, R.R. COVID-19—Navigating the Uncharted. N. Engl. J. Med. 2020, 382, 1268–1269. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Rod, J.E.; Oviedo-Trespalacios, O.; Cortes-Ramirez, J. A brief-review of the risk factors for COVID-19 severity. Rev. Saude Publica 2020, 54, 60. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yuan, X.; Xiao, J.; Zhong, Q.; Yang, C.; Liu, B.; Cai, Y.; Lu, Z.; Wang, J.; Wang, Y.; et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: A multicentre, retrospective, cohort study. Lancet Oncol. 2020, 21, 893–903. [Google Scholar] [CrossRef]
- Au, L.; Boos, L.A.; Swerdlow, A.; Byrne, F.; Shepherd, S.T.C.; Fendler, A.; Turajlic, S.; on behalf of CAPTURE investigators. Cancer, COVID-19, and Antiviral Immunity: The CAPTURE Study. Cell 2020, 183, 4–10. [Google Scholar] [CrossRef]
- Goldman, J.D.; Ascierto, P.A. Perspectives on COVID-19 and cancer immunotherapy: A review series. J. Immunother. Cancer 2021, 9, e002489. [Google Scholar] [CrossRef]
- Bondhopadhyay, B.; Sisodiya, S.; Chikara, A.; Khan, A.; Tanwar, P.; Afroze, D.; Singh, N.; Agrawal, U.; Mehrotra, R.; Hussain, S. Cancer immunotherapy: A promising dawn in cancer research. Am. J. Blood Res. 2020, 10, 375–385. [Google Scholar]
- Veldman, J.; Visser, L.; Berg, A.V.D.; Diepstra, A. Primary and acquired resistance mechanisms to immune checkpoint inhibition in Hodgkin lymphoma. Cancer Treat. Rev. 2020, 82, 101931. [Google Scholar] [CrossRef] [Green Version]
- Kuzume, A.; Chi, S.; Yamauchi, N.; Minami, Y. Immune-Checkpoint Blockade Therapy in Lymphoma. Int. J. Mol. Sci. 2020, 21, 5456. [Google Scholar] [CrossRef]
- Hatic, H.; Sampat, D.; Goyal, G. Immune checkpoint inhibitors in lymphoma: Challenges and opportunities. Ann. Transl. Med. 2021, 9, 1037. [Google Scholar] [CrossRef]
- Eyre, T.A.; Collins, G.P. Immune checkpoint inhibition in lymphoid disease. Br. J. Haematol. 2015, 170, 291–304. [Google Scholar] [CrossRef]
- Thanarajasingam, G.; Thanarajasingam, U.; Ansell, S.M. Immune checkpoint blockade in lymphoid malignancies. FEBS J. 2016, 283, 2233–2244. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Gandhi, L.; Garassino, M.C. Pembrolizumab plus Chemotherapy in Lung Cancer. N. Engl. J. Med. 2018, 379, e18. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Wu, Y.L.; Lu, S.; Cheng, Y.; Zhou, C.; Wang, J.; Mok, T.; Zhang, L.; Tu, H.Y.; Wu, L.; Feng, J.; et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J. Thorac. Oncol. 2019, 14, 867–875. [Google Scholar] [CrossRef]
- Reck, M.; Mok, T.S.K.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir. Med. 2019, 7, 387–401. [Google Scholar] [CrossRef]
- Nishio, M.; Felip, E.; Orlov, S.; Park, K.; Yu, C.J.; Tsai, C.M.; Cobo, M.; McKeage, M.; Su, W.C.; Mok, T.; et al. Final Overall Survival and Other Efficacy and Safety Results From ASCEND-3: Phase II Study of Ceritinib in ALKi-Naive Patients With ALK-Rearranged NSCLC. J. Thorac. Oncol. 2020, 15, 609–617. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, L.L.; Chen, J.H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal. Transduct. Target Ther. 2019, 4, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, L.; Thienpont, B.; Zhao, L.; Wolf, D.; Pircher, A. Overcoming immunotherapy resistance in non-small cell lung cancer (NSCLC)—Novel approaches and future outlook. Mol. Cancer 2020, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K. Immune Checkpoint Inhibitors for Lung Cancer Treatment: A Review. J. Clin. Med. 2020, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Liu, Y.T.; Sun, Z.J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef]
- Maleki Vareki, S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 2018, 6, 157. [Google Scholar] [CrossRef]
- Heong, V.; Ngoi, N.; Tan, D.S. Update on immune checkpoint inhibitors in gynecological cancers. J. Gynecol. Oncol. 2017, 28, e20. [Google Scholar] [CrossRef] [Green Version]
- Strasner, A.; Karin, M. Immune Infiltration and Prostate Cancer. Front. Oncol. 2015, 5, 128. [Google Scholar] [CrossRef]
- Martinez-Bosch, N.; Vinaixa, J.; Navarro, P. Immune Evasion in Pancreatic Cancer: From Mechanisms to Therapy. Cancers 2018, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Giustarini, G.; Pavesi, A.; Adriani, G. Nanoparticle-Based Therapies for Turning Cold Tumors Hot: How to Treat an Immunosuppressive Tumor Microenvironment. Front. Bioeng. Biotechnol. 2021, 9, 689245. [Google Scholar] [CrossRef]
- Sha, Z.; Chang, K.; Mi, J.; Liang, Z.; Hu, L.; Long, F.; Shi, H.; Lin, Z.; Wang, X.; Pei, X. The impact of the COVID-19 pandemic on lung cancer patients. Ann. Palliat Med. 2020, 9, 3373–3378. [Google Scholar] [CrossRef]
- Calabro, L.; Rossi, G.; Covre, A.; Morra, A.; Maio, M. COVID and Lung Cancer. Curr. Oncol. Rep. 2021, 23, 134. [Google Scholar] [CrossRef]
- Passaro, A.; Bestvina, C.; Velez Velez, M.; Garassino, M.C.; Garon, E.; Peters, S. Severity of COVID-19 in patients with lung cancer: Evidence and challenges. J. Immunother. Cancer 2021, 9, e002266. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Grivas, P.; Khaki, A.R.; Wise-Draper, T.M.; French, B.; Hennessy, C.; Hsu, C.Y.; Shyr, Y.; Li, X.; Choueiri, T.K.; Painter, C.A.; et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: A report from the COVID-19 and Cancer Consortium. Ann. Oncol. 2021, 32, 787–800. [Google Scholar] [CrossRef]
- Shah, A.S.; Wong, A.W.; Hague, C.J.; Murphy, D.T.; Johnston, J.C.; Ryerson, C.J.; Carlsten, C. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax 2021, 76, 402–404. [Google Scholar] [CrossRef]
- Drake, T.M.; Docherty, A.B.; Harrison, E.M.; Quint, J.K.; Adamali, H.; Agnew, S.; Babu, S.; Barber, C.M.; Barratt, S.; Bendstrup, E.; et al. Outcome of Hospitalization for COVID-19 in Patients with Interstitial Lung Disease. An International Multicenter Study. Am. J. Respir. Crit. Care Med. 2020, 202, 1656–1665. [Google Scholar] [CrossRef]
- Bakouny, Z.; Labaki, C.; Grover, P.; Awosika, J.; Gulati, S.; Hsu, C.Y.; Alimohamed, S.I.; Bashir, B.; Berg, S.; Bilen, M.A.; et al. Interplay of Immunosuppression and Immunotherapy Among Patients With Cancer and COVID-19. JAMA Oncol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, J.L. Host Immune Response to Influenza A Virus Infection. Front. Immunol. 2018, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wei, X.; Guan, J.; Qin, S.; Wang, Z.; Lu, H.; Qian, J.; Wu, L.; Chen, Y.; Chen, Y.; et al. COVID-19 pneumonia: CD8(+) T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin. Immunol. 2020, 218, 108516. [Google Scholar] [CrossRef]
- Garassino, M.C.; Ribas, A. At the Crossroads: COVID-19 and Immune-Checkpoint Blockade for Cancer. Cancer Immunol. Res. 2021, 9, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litiere, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabro, L.; Peters, S.; Soria, J.C.; Di Giacomo, A.M.; Barlesi, F.; Covre, A.; Altomonte, M.; Vegni, V.; Gridelli, C.; Reck, M.; et al. Challenges in lung cancer therapy during the COVID-19 pandemic. Lancet Respir. Med. 2020, 8, 542–544. [Google Scholar] [CrossRef]
- Acharya, D.; Lee, K.; Lee, D.S.; Lee, Y.S.; Moon, S.S. Mortality Rate and Predictors of Mortality in Hospitalized COVID-19 Patients with Diabetes. Healthcare 2020, 8, 338. [Google Scholar] [CrossRef]
- Salunke, A.A.; Nandy, K.; Pathak, S.K.; Shah, J.; Kamani, M.; Kottakota, V.; Thivari, P.; Pandey, A.; Patel, K.; Rathod, P.; et al. Impact of COVID -19 in cancer patients on severity of disease and fatal outcomes: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020, 14, 1431–1437. [Google Scholar] [CrossRef]
- Rogiers, A.; Pires da Silva, I.; Tentori, C.; Tondini, C.A.; Grimes, J.M.; Trager, M.H.; Nahm, S.; Zubiri, L.; Manos, M.; Bowling, P.; et al. Clinical impact of COVID-19 on patients with cancer treated with immune checkpoint inhibition. J. Immunother. Cancer 2021, 9, e001931. [Google Scholar] [CrossRef]
- Yekeduz, E.; Arzu Yasar, H.; Utkan, G.; Urun, Y. A systematic review: Role of systemic therapy on treatment and prevention of brain metastasis in renal cell carcinoma. J. Oncol. Pharm. Pract. 2020, 26, 972–981. [Google Scholar] [CrossRef]
- Cao, C.; Gan, X.; Hu, X.; Su, Y.; Zhang, Y.; Peng, X. Association of active immunotherapy with outcomes in cancer patients with COVID-19: A systematic review and meta-analysis. Aging 2022, 14, 2062–2080. [Google Scholar] [CrossRef]
- Liu, H.; Yang, D.; Chen, X.; Sun, Z.; Zou, Y.; Chen, C.; Sun, S. The effect of anticancer treatment on cancer patients with COVID-19: A systematic review and meta-analysis. Cancer Med. 2021, 10, 1043–1056. [Google Scholar] [CrossRef]
- Patanavanich, R.; Glantz, S.A. Smoking is associated with worse outcomes of COVID-19 particularly among younger adults: A systematic review and meta-analysis. BMC Public Health 2021, 21, 1554. [Google Scholar] [CrossRef]
- Zukin, M.; Barrios, C.H.; Pereira, J.R.; Ribeiro Rde, A.; Beato, C.A.; do Nascimento, Y.N.; Murad, A.; Franke, F.A.; Precivale, M.; Araujo, L.H.; et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J. Clin. Oncol. 2013, 31, 2849–2853. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Okwan-Duodu, D.; Basho, R.; Cui, X. COVID-19 in cancer patients: Risk, clinical features, and management. Cancer Biol. Med. 2020, 17, 519–527. [Google Scholar] [CrossRef]
- von Lilienfeld-Toal, M.; Vehreschild, J.J.; Cornely, O.; Pagano, L.; Compagno, F.; Group, E.H.A.I.D.S.W.; Hirsch, H.H. Frequently asked questions regarding SARS-CoV-2 in cancer patients-recommendations for clinicians caring for patients with malignant diseases. Leukemia 2020, 34, 1487–1494. [Google Scholar] [CrossRef]
- Alhalabi, O.; Subbiah, V. Managing Cancer Care during the COVID-19 Pandemic and Beyond. Trends Cancer 2020, 6, 533–535. [Google Scholar] [CrossRef]
- Russano, M.; Citarella, F.; Napolitano, A.; Dell’Aquila, E.; Cortellini, A.; Pantano, F.; Vincenzi, B.; Tonini, G.; Santini, D. COVID-19 pneumonia and immune-related pneumonitis: Critical issues on differential diagnosis, potential interactions, and management. Expert Opin. Biol. Ther. 2020, 20, 959–964. [Google Scholar] [CrossRef]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]



| Patient Characteristics | Treatment | |||
|---|---|---|---|---|
| Sample | Chemotherapy (n = 60) | Immunotherapy (n = 61) | t/Χ2 | |
| Sociodemographics | ||||
| Age (M/SD) | 63.7 (11.6) | 65.1 (12.9) | 62.3 (10.0) | 1.4 |
| Sex | ||||
| Female | 51.0 (42.2%) | 28.0 (46.7%) | 23.0 (37.7%) | |
| Male | 70.0 (57.9%) | 32.0 (53.3%) | 38.0 (62.3%) | 1.0 |
| Race/Ethnicity | ||||
| Black | 35.0(28.93%) | 16 (26.67%) | 19.0 (31.2%) | |
| White | 84.0 (69.4%) | 44.0 (73.3%) | 40.0 (65.6%) | |
| Asian/Latinx/Other | 2.0 (0.2%) | 0.0 (0.00%) | 2.0 (3.3%) | 2.4 |
| Clinical Factors | ||||
| Comorbidities | ||||
| 0 | 4.0 (3.3%) | 1.0 (1.7%) | 3.0 (4.9%) | |
| 1–2 | 15.0 (12.4%) | 7.0 (11.4%) | 8.0 (13.1%) | |
| 3–5 | 64.0 (52.9%) | 30.0 (50.0%) | 34.0 (55.7%) | |
| >5 | 38.0 (31.4%) | 22.0 (36.4%) | 16.0 (26.2%) | 2.3 |
| ECOG | ||||
| 0–1 | 96.0 (79.3%) | 48.0 (80.0%) | 48.0 (78.7%) | |
| 2–4 | 25.0 (20.7%) | 12.0 (20.0%) | 13.0 (21.3%) | 0.03 |
| Cancer Type | ||||
| Lung | 40.0 (33.1%) | 20.0 (33.3%) | 20.0 (33.3%) | |
| Liver | 15.0 (12.4%) | 7.0 (11.7%) | 8.0 (13.1%) | |
| Renal | 10.0 (8.3%) | 3.0 (5.0%) | 7.0 (11.4%) | |
| Head and Neck | 11.0 (9.1%) | 6.0 (10.0%) | 5.0 (8.2%) | |
| Other | 45.0 (37.2%) | 24.0 (40.0%) | 21.0 (34.4%) | 2.0 |
| Symptoms at COVID-19 Diagnosis | ||||
| Fever | 19.0 (21.6%) | 12.0 (23.1%) | 7.0 (19.4%) | 1.1 |
| Cough | 24.0 (27.3%) | 12.0 (23.1%) | 12.0 (33.3%) | 0.002 |
| Dyspnea | 45.0 (51.1%) | 28.0 (53.9%) | 17.0 (47.2%) | 4.6 * |
| PD-L1 Expression (M/SD) | 13.1 (26.6) | 11.4 (27.2) | 14.9 (26.6) | 0.7 |
| Immunotherapy | ||||
| PD-1/PD-L1 | -- | 58.0 (95.1%) | ||
| CTLA-4 | -- | 1.0 (1.6%) | ||
| Combined | -- | 2.0 (3.3%) | ||
| Admission | ||||
| Home | 42.0 (34.7%) | 20.0 (33.3%) | 22.0 (36.1%) | |
| Floor | 70.0 (57.9%) | 37.0 (61.7%) | 33.0 (54.1%) | |
| Intensive Care | 9.0 (7.4%) | 3.0 (5.0%) | 6.0 (9.8%) | 1.3 |
| Oxygen Use | 28.0 (23.1%) | 14.0 (23.3%) | 14.0 (23.0%) | 0.003 |
| Mechanical Ventilation | 2.0 (3.3%) | 0.0 (0.0%) | 2.0 (1.7%) | 2.1 |
| Treatment | ||||
| Steroids | 31.0 (25.62%) | 12.0 (20.0%) | 19.0 (31.2%) | 2.0 |
| Antiviral | 18.0 (14.9%) | 9.0 (15.0%) | 9.0 (14.8%) | 0.001 |
| Antibiotics | 28.0 (23.1%) | 14.0 (23.3%) | 14.0 (23.0%) | 0.003 |
| Mortality | 23.0 (19.0%) | 8.0 (13.3%) | 15.0 (24.6%) | 2.5 |
| Patient Characteristics | Not a Smoker | Current Smoker | Former Smoker | X2/t/F |
|---|---|---|---|---|
| Clinical Factors | ||||
| Admission | ||||
| Floor | 15.0/44.1% | 12.0/60.0% | 43.0/64.2% | |
| Home | 18.0/52.9% | 7.0/35.0% | 17.0/25.4% | |
| MICU | 1.0/2.9% | 1.0/5.0% | 7/10.45% | 8.4 |
| Days from Cancer Dx to COVID-19 | 629.6/652.3 | 342.2/454.0 | 585.1/821.5 | 1.1 |
| Days from end of COVID-19 treatment | 159.2/128.3 | 196.7/126.1 | 260.7/204.7 | 8.6 * |
| Oxygen Use | 7.0/20.6% | 4.0/20.0% | 17.0/25.3% | 5.0 |
| Treatment | ||||
| Steroids | 8.0/25.5% | 3.0/15.0% | 20.0/29.9% | 1.9 |
| Antiviral | 8.0/14.7% | 2.0/10.0% | 11.0/16.4% | 0.5 |
| Antibiotics | 5.0/14.7% | 4/20.00% | 19/28.36% | 2.5 |
| Death | 6.0/17.7% | 3.0/15.0% | 14.0/20.9% | 0.4 |
| Patient Characteristics | COVID-19 Vaccination | No COVID-19 Vaccination | X2 or t |
|---|---|---|---|
| Clinical Factors | |||
| Admission | |||
| Floor | 40.0/57.1% | 30.0/58.8% | |
| Home | 29.0/41.4% | 13.0/25.5% | |
| MICU | 1.0/1.4% | 8.0/15.7% | 10.2 ** |
| Days from Cancer Dx to COVID-19 | 510.3/696.4 | 622.1/722.3 | 0.8 |
| Days from end of COVID-19 treatment | 230.0/144.6 | 126.7/127.5 | 4.1 *** |
| Oxygen Use | 19.0/37.2% | 9.0/12.9% | 11.3 * |
| Treatment | |||
| Steroids | 15.0/21.4% | 16.0/31.4% | 1.5 |
| Antiviral | 6.0/8.6% | 12.0/23.5% | 5.2 * |
| Antibiotics | 12.0/17.1% | 16.0/31.4% | 3.4 |
| Death | 5.0/7.1% | 18.0/35.3% | 15.2 *** |
| Patient Characteristics | Admission | ICU | Death |
|---|---|---|---|
| Immunotherapy—61 | 28.0 (45.9%) | 6.0 (9.8%) | 15.0 (24.6%) ^ |
| Chemotherapy—60 | 23.0 (38.3%) | 3.0 (5.0%) | 8.0 (13.3%) ^ |
| Sociodemographics | |||
| Age (M/SD) | 64.6 (8.7) | 63.0 (11.4) | 63.0 (11.4) |
| Sex | |||
| Female | 44.0 (62.9%) | 7.0 (77.8%) | 8.0 (34.8%) |
| Male | 26.0 (51.0%) | 2.0 (22.2%) | 15.0 (65.2%) |
| Race/Ethnicity | |||
| Black | 26.0 (37.1%) | 2.0 (22.2%) | 6.0 (26.1%) |
| White | 44.0 (62.9%) | 6.0 (22.2%) | 16.0 (69.6%) |
| Asian/Latinx/Other | 0.0 (0.0%) * | 1.0 (11.11%) | 1.0 (4.35%) |
| Clinical Factors | |||
| Comorbidities | |||
| 0 | 1.0 (1.4%) | 0.0 (0.0%) | 0.0 (0.0%) |
| 1–2 | 10.0 (14.3%) | 2.0 (22.2%) | 4.0 (17.4%) |
| 3–5 | 38.0 (54.3.0%) | 5.0 (55.6%) | 10.0 (43.5%) |
| >5 | 21.0 (30.0%) | 2.0 (22.2%) | 9.0 (39.1%) |
| ECOG | 1.0 (0.8) | 1.7 (0.7) | 1.5 (0.8) |
| Symptoms at COVID-19 Diagnosis | |||
| Fever | 11.0 (21.6%) ** | 7.0 (77.8%) ** | 33.0 (33.4%) |
| Cough | 5.0 (19.2%) | 1.0 (11.1%) | 4.0 (17.4%) |
| Dyspnea | 8.0 (15.7%) | 4.0 (44.4%) * | 4.0 (17.4%) |
| PD-L1 Expression (M/SD) | 12.8 (27.0) | 19.1 (33.7) | 13.0 (23.6) |
| Immunotherapy | |||
| PD-1/PD-L1 | 31.0 (93.9%) | 6.0 (100.0%) | 14.0 (93.3%) |
| CTLA-4 | 0.0 (0.0%) | -- | 1 (6.7%) |
| Combined | 2.0 (6.1%) | -- | 0 (0.00%) |
| AST | 40.9 (48.3) | 99.1 (94.6) ** | 50.0 (65.4) |
| LDH | 341.1 (317.9) | 564.3 (436.3) | 381.1 (327.0) |
| Oxygen Use | 19.0 (27.7%) | 8.0 (88.9%) *** | 12.0 (52.2%) *** |
| Treatment | |||
| Steroids | 27.0 (34.3%) | 7.0 (77.8%) * | 10.0 (43.5%) * |
| Antiviral | 12.0 (17.1%) | 6.0 (66.7%) *** | 7.0 (30.4%) * |
| Antibiotics | 21.0 (30.0%) * | 7.0 (77.8%) *** | 8.0 (34.8%) |
| Patient Characteristic | Outcomes | ||
|---|---|---|---|
| Admission | ICU | Death | |
| Cancer | |||
| Lung | 27/38.57% | 4/44.44% | 7/30.43% |
| Liver | 8/11.43% | 1/11.11% | 0/0.00% |
| Renal | 9/12.86% | 0/0.00% | 3/13.04% |
| Head and Neck | 2/2.86% | 1/11.11% | 1/4.35% |
| Other | 24/34.28% * | 3/33.34% | 12/52.18% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatic, H.; Hearld, K.R.; Das, D.; Deshane, J. Clinical Outcomes in COVID-19 Patients Treated with Immunotherapy. Cancers 2022, 14, 5954. https://doi.org/10.3390/cancers14235954
Hatic H, Hearld KR, Das D, Deshane J. Clinical Outcomes in COVID-19 Patients Treated with Immunotherapy. Cancers. 2022; 14(23):5954. https://doi.org/10.3390/cancers14235954
Chicago/Turabian StyleHatic, Haris, Kristine R. Hearld, Devika Das, and Jessy Deshane. 2022. "Clinical Outcomes in COVID-19 Patients Treated with Immunotherapy" Cancers 14, no. 23: 5954. https://doi.org/10.3390/cancers14235954
APA StyleHatic, H., Hearld, K. R., Das, D., & Deshane, J. (2022). Clinical Outcomes in COVID-19 Patients Treated with Immunotherapy. Cancers, 14(23), 5954. https://doi.org/10.3390/cancers14235954