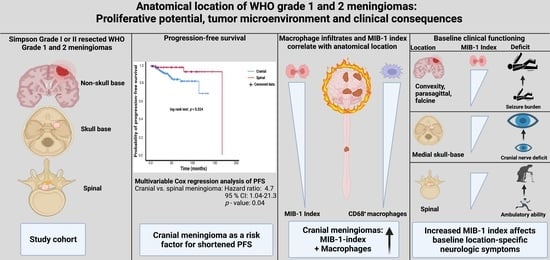
Proliferative Potential, and Inflammatory Tumor Microenvironment in Meningioma Correlate with Neurological Function at Presentation and Anatomical Location—From Convexity to Skull Base and Spine
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Data Recording
2.4. Histopathology
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. MIB-1 Index in Different Anatomic Locations of Meningioma
3.3. Density of CD68+ Macrophage Infiltrates
3.4. Anatomic Meningioma Location in the Prediction of Progression-Free Survival
3.5. Clinical Implications of MIB-1 Index in Different Anatomical Locations
3.6. Postoperative Course and Postoperative Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2015, 17 (Suppl. S4), iv1–iv62. [Google Scholar] [CrossRef] [PubMed]
- Domingues, P.H.; Sousa, P.; Otero, Á.; Gonçalves, J.M.; Ruiz, L.; de Oliveira, C.; Lopes, M.C.; Orfao, A.; Tabernero, M.D. Proposal for a new risk stratification classification for meningioma based on patient age, WHO tumor grade, size, localization, and karyotype. Neuro Oncol. 2014, 16, 735–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, G.A.; Gogou, P.; Markoula, S.; Kyritsis, A.P. Management of meningiomas. Clin. Neurol. Neurosurg. 2010, 112, 177–182. [Google Scholar] [CrossRef]
- Kalasauskas, D.; Kronfeld, A.; Renovanz, M.; Kurz, E.; Leukel, P.; Krenzlin, H.; Brockmann, M.A.; Sommer, C.J.; Ringel, F.; Keric, N. Identification of High-Risk Atypical Meningiomas According to Semantic and Radiomic Features. Cancers 2020, 12, 2942. [Google Scholar] [CrossRef]
- Barresi, V.; Lionti, S.; Caliri, S.; Caffo, M. Histopathological features to define atypical meningioma: What does really matter for prognosis? Brain Tumor Pathol. 2018, 35, 168–180. [Google Scholar] [CrossRef]
- Barrett, O.C.; Hackney, J.R.; McDonald, A.M.; Willey, C.D.; Bredel, M.; Fiveash, J.B. Pathologic Predictors of Local Recurrence in Atypical Meningiomas Following Gross Total Resection. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 453–459. [Google Scholar] [CrossRef]
- Chen, X.; Wang, G.; Zhang, J.; Zhang, G.; Lin, Y.; Lin, Z.; Gu, J.; Kang, D.; Ding, C. A Novel Scoring System Based on Pre-operative Routine Blood Test in Predicting Prognosis of Atypical Meningioma. Front. Oncol. 2020, 10, 1705. [Google Scholar] [CrossRef]
- Vranic, A.; Popovic, M.; Cör, A.; Prestor, B.; Pizem, J. Mitotic Count, Brain Invasion, and Location Are Independent Predictors of Recurrence-Free Survival in Primary Atypical and Malignant Meningiomas: A Study of 86 Patients. Neurosurgery 2010, 67, 1124–1132. [Google Scholar] [CrossRef] [Green Version]
- Oya, S.; Kawai, K.; Nakatomi, H.; Saito, N. Significance of Simpson grading system in modern meningioma surgery: Integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J. Neurosurg. 2012, 117, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Kim, K.H.; Lee, E.H.; Lee, Y.M.; Lee, S.-H.; Kim, H.D.; Kim, Y.Z. Results of immunohistochemical staining for cell cycle regulators predict the recurrence of atypical meningiomas. J. Neurosurg. 2014, 121, 1189–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Song, S.Y.; Jiang, J.B.; Wang, T.J.; Yan, C.X. The prognostic role of Ki-67/MIB-1 in meningioma: A systematic review with meta-analysis. Medicine 2020, 99, e18644, discussion 44. [Google Scholar] [CrossRef] [PubMed]
- Wach, J.; Brandecker, S.; Güresir, A.; Schuss, P.; Vatter, H.; Güresir, E. The impact of the MIB-1 index on facial nerve outcomes in vestibular schwannoma surgery. Acta Neurochir. 2020, 162, 1205–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerdes, J.; Schwab, U.; Lemke, H.; Stein, H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 1983, 31, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Wach, J.; Lampmann, T.; Güresir, Á.; Schuss, P.; Vatter, H.; Herrlinger, U.; Becker, A.; Hölzel, M.; Toma, M.; Güresir, E. FORGE: A Novel Scoring System to Predict the MIB-1 Labeling Index in Intracranial Meningiomas. Cancers 2021, 13, 3643. [Google Scholar] [CrossRef]
- Wach, J.; Lampmann, T.; Güresir, Á.; Vatter, H.; Becker, A.; Hölzel, M.; Toma, M.; Güresir, E. Combining FORGE Score and Histopathological Diagnostic Criteria of Atypical Meningioma Enables Risk Stratification of Tumor Progression. Diagnostics 2021, 11, 2011. [Google Scholar] [CrossRef]
- Proctor, D.T.; Huang, J.; Lama, S.; Albakr, A.; Van Marle, G.; Sutherland, G.R. Tumor-associated macrophage infiltration in meningioma. Neurooncol. Adv. 2019, 1, vdz018. [Google Scholar] [CrossRef]
- Meling, T.R.; Da Broi, M.; Scheie, D.; Helseth, E. Skull base versus non-skull base meningioma surgery in the elderly. Neurosurg. Rev. 2019, 42, 163–173. [Google Scholar] [CrossRef]
- Cornelius, J.F.; Slotty, P.J.; Steiger, H.J.; Hänggi, D.; Polivka, M.; George, B. Malignant potential of skull base versus non-skull base meningiomas: Clinical series of 1663 cases. Acta Neurochir. 2013, 155, 407–413. [Google Scholar] [CrossRef]
- Wu, A.; Garcia, M.A.; Magill, S.T.; Chen, W.; Vasudevan, H.N.; Perry, A.; Theodosopoulos, P.V.; McDermott, M.W.; Braunstein, S.E.; Raleigh, D.R. Presenting Symptoms and Prognostic Factors for Symptomatic Outcomes Following Resection of Meningioma. World Neurosurg. 2018, 111, e149–e159. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Shin, Y.S.; Kim, J.H.; Choi, M.; Yoo, S.H. Differences in Type Composition of Symptom Clusters as Predictors of Quality of Life in Patients with Meningioma and Glioma. World Neurosurg. 2017, 98, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Coons, S.W.; Johnson, P.C. Regional heterogeneity in the proliferative activity of human gliomas as measured by the Ki-67 labeling index. J. Neuropathol. Exp. Neurol. 1993, 52, 609–618. [Google Scholar] [CrossRef]
- Antinheimo, J.; Haapasalo, H.; Haltia, M.; Tatagiba, M.; Thomas, S.; Brandis, A.; Sainio, M.; Carpen, O.; Samii, M.; Jääskeläinen, J. Proliferation potential and histological features in neurofibromatosis 2-associated and sporadic meningiomas. J. Neurosurg. 1997, 87, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef] [Green Version]
- Wach, J.; Banat, M.; Schuss, P.; Güresir, E.; Vatter, H.; Scorzin, J. Age at Diagnosis and Baseline Myelomalacia Sign Predict Functional Outcome After Spinal Meningioma Surgery. Front. Surg. 2021, 8, 682930. [Google Scholar] [CrossRef]
- Maiuri, F.; Mariniello, G.; Guadagno, E.; Barbato, M.; Corvino, S.; Del Basso De Caro, M. WHO grade, proliferation index, and progesterone receptor expression are different according to the location of meningioma. Acta Neurochir. 2019, 161, 2553–2561. [Google Scholar] [CrossRef]
- Wach, J.; Apallas, S.; Schneider, M.; Güresir, A.; Schuss, P.; Herrlinger, U.; Vatter, H.; Güresir, E. Baseline Serum C-Reactive Protein and Plasma Fibrinogen-Based Score in the Prediction of Survival in Glioblastoma. Front. Oncol. 2021, 11, 653614. [Google Scholar] [CrossRef]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control. 1999, 27, 97–132; discussion 96; quiz 133-4. [Google Scholar] [CrossRef]
- Majores, M.; Schick, V.; Engels, G.; Fassunke, J.; Elger, C.E.; Schramm, J.; Blumcke, I.; Becker, A.J. Mutational and immunohistochemical analysis of ezrin-, radixin-, moesin (ERM) molecules in epilepsy-associated glioneuronal lesions. Acta Neuropathol. 2005, 110, 537–546. [Google Scholar] [CrossRef]
- Majores, M.; von Lehe, M.; Fassunke, J.; Schramm, J.; Becker, A.J.; Simon, M. Tumor recurrence and malignant progression of gangliogliomas. Cancer 2008, 113, 3355–3363. [Google Scholar] [CrossRef]
- Schneider, M.; Borger, V.; Güresir, A.; Becker, A.; Vatter, H.; Schuss, P.; Güresir, E. High Mib-1-score correlates with new cranial nerve deficits after surgery for frontal skull base meningioma. Neurosurg. Rev. 2021, 44, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Lemée, J.M.; Corniola, M.V.; Meling, T.R. Benefits of re-do surgery for recurrent intracranial meningiomas. Sci. Rep. 2020, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Haller, B.; Ulm, K.; Hapfelmeier, A. A Stimulation Study Comparing Different Statistical Approaches for the identification of Predictive Biomarkers. Comput. Math. Methods Med. 2009, 2019, 7037230. [Google Scholar]
- Apra, C.; Peyre, M.; Kalamarides. Current treatment options for meningioma. Expert Rev. Neurother. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Dundamadappa, S.K.; Thangasamy, S.; Flood, T.; Moser, R.; Smith, T.; Cauley, K.; Takhtani, D. Correlation of apparent diffusion coefficient with Ki-67 proliferation index in grading meningioma. AJR Am. J. Roentgenol. 2014, 202, 1303–1308. [Google Scholar] [CrossRef]
- Pavelin, S.; Becic, K.; Forempoher, G.; Mrklic, I.; Pogorelic, Z.; Titlic, M.; Andelinovic, S. Expression of Ki-67 and p53 in meningiomas. Neoplasma 2013, 60, 480–485. [Google Scholar] [CrossRef] [Green Version]
- Roser, F.; Nakamura, M.; Bellinzona, M.; Ritz, R.; Ostertag, H.; Tatagiba, M.S. Proliferation potential of spinal meningiomas. Eur. Spine J. 2006, 15, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Metellus, P.; Nanni, I.; Dussert, C.; Trinkhaus, M.; Fuentes, S.; Chinot, O.; Ouafik, L.H.; Fina, F.; Dufour, H.; Figarella-Branger, D.; et al. Prognostic implications of biologic markers in intracranial meningiomas: 120 cases. Neurochirurgie 2008, 54, 750–756. [Google Scholar] [CrossRef]
- Pravdenkova, S.; Al-Mefty, O.; Sawyer, J.; Husain, M. Progesterone and estrogen receptors: Opposing prognostic indicators in meningiomas. J. Neurosurg. 2006, 105, 183–202. [Google Scholar] [CrossRef] [Green Version]
- Roser, F.; Nakamura, M.; Bellinzona, M.; Rosahl, S.K.; Ostertag, H.; Samii, M. The prognostic value of progesterone receptor status in meningiomas. J. Clin. Pathol. 2004, 57, 1033–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roser, F.; Nakamura, M.; Ritz, R.; Bellinzona, M.; Dietz, K.; Samii, M.; Tatagiba, M.S. Proliferation and progesterone receptor status in benign meningiomas are not age dependent. Cancer 2005, 104, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Wolfsberger, S.; Doostkam, S.; Boecher-Schwarz, H.G.; Roessler, K.; van Trotsenburg, M.; Hainfellner, J.A. Progesterone-receptor index in meningiomas: Correlation with clinico-pathological parameters and review of the literature. Neurosurg. Rev. 2004, 27, 238–345. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Alafaci, C.; Caffo, M.; Barresi, G.; Tuccari, G. Clinicopathological characteristics, hormone receptor status and matrix metallo-proteinase-9 (MMP-9) immunohistochemical expression in spinal meningiomas. Pathol. Res. Pract. 2012, 208, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, F.; Del Basso De Caro, M.L.; de Divitiis, O.; Guadagno, E.; Mariniello, G. Recurrence of spinal meningiomas: Analysis of the risk factors. Br. J. Neurosurg. 2020, 34, 569–574. [Google Scholar] [CrossRef]
- Maiuri, F.; Mariniello, G.; de Divitiis, O.; Esposito, F.; Guadagno, E.; Teodonno, G.; Barbato, M.; Del Basso de Caro, M. Progesteron Receptor Expression in Meningiomas: Pathological and Prognostic Implications. Front. Oncol. 2021, 11, 511218. [Google Scholar] [CrossRef]
- Todo, T.; Adams, E.F.; Rafferty, B.; Fahlbusch, R.; Dingermann, T.; Werner, H. Secretion of interleukin-6 by human meningioma cells: Possible autocrine inhibitory regulation of neoplastic cell growth. J. Neurosurg. 1994, 81, 394–401. [Google Scholar] [CrossRef]
- Wong, L.Y.F.; Leung, R.Y.H.; Ong, K.L.; Cheung, B.M.Y. Plasma levels of fibrinogen and C-reactive protein are related to interleukin-6 gene -572C>G polymorphism in subjects with and without hypertension. J. Hum. Hypertens. 2007, 21, 875–882. [Google Scholar] [CrossRef]
- Salpietro, F.M.; Alafaci, C.; Lucerna, S.; Iacopino, D.G.; Tomasello, F. Do spinal menignomas penetrate the pial layer? Correlation between magnetic resonance imaging and microsurgical findings and intracranial tumor interfaces. Neurosurgery 1997, 41, 254–257. [Google Scholar] [CrossRef]
- Solero, C.L.; Fornari, M.; Giombini, S.; Lasio, G.; Oliveri, G.; Cimino, C.; Pluchino, F. Spinal meningiomas: Review of 174 operated cases. Neurosurgery 1989, 25, 153–160. [Google Scholar] [CrossRef]
- Klekamp, J.; Samii, M. Surgical results for spinal meningiomas. Surg. Neurol. 1999, 52, 552–562. [Google Scholar] [CrossRef]
- Nakamura, M.; Tsuji, O.; Fujiyoshi, K.; Hosogane, N.; Watanabe, K.; Tsuji, T.; Ishii, K.; Toyama, Y.; Chiba, K.; Matsumoto, M. Long-term surgical outcomes of spinal meningiomas. Spine 2012, 37, E617–E623. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Bir, S.C.; Maiti, T.K.; Konar, S.K.; Missios, K.; Guthikonda, B. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J. Neurosurg. 2017, 126, 201–211. [Google Scholar] [CrossRef]
- Barber, S.M.; Konakondla, S.; Nakhla, J.; Fridley, J.S.; Xia, J.; Oyelese, A.A.; Telfeian, A.E.; Gokaslan, Z.L. Oncologic benefits of dural resection in spinal meningiomas: A meta-analysis of Simpson grades and recurrence rates. J. Neurosurg. Spine 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Kanungo, I.; Sudhir, S.; Chen, J.S.; Raleigh, D.R.; Magill, S.T.; McDermott, M.W.; Aghi, M.K. WHO Grade I Meningioma Recurrence: Identifying High Risk Patients Using Histopathological Features and the MIB-1 Index. Front. Oncol. 2020, 10, 1522. [Google Scholar] [CrossRef] [PubMed]
- Ohba, S.; Kobayashi, M.; Horiguchi, T.; Onozuka, S.; Yoshida, K.; Ohira, T.; Kawase, T. Long-term surgical outcome and biological prognostic factors in patients with skull base meningiomas. J. Neurosurg. 2011, 114, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Soyuer, S.; Chang, E.L.; Selek, U.; Shi, W.; Maor, M.H.; DeMonte, F. Radiotherapy after surgery for benign cerebral meningioma. Radiother. Oncol. 2004, 71, 85–90. [Google Scholar] [CrossRef]
- Park, S.; Cha, Y.J.; Suh, S.H.; Lee, I.J.; Lee, K.S.; Hong, C.K.; Kim, J.W. Risk group-adapted adjuvant radiotherapy for WHO grade I and II skull base meningioma. J. Cancer Res. Clin. Oncol. 2019, 145, 1351–1360. [Google Scholar] [CrossRef]
- Oya, S.; Ikawa, F.; Ichihara, N.; Wanibuchi, M.; Akiyama, Y.; Nakatomi, H.; Mikuni, N.; Narita, Y. Effect of adjuvant radiotherapy after subtotal resection for WHO grade I meningioma: A propensity score matching analysis of the Brain Tumor Registry of Japan. J. Neurooncol. 2021, 153, 351–360. [Google Scholar] [CrossRef]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef] [Green Version]
- Mirian, C.; Skyrman, S.; Bartek, J., Jr.; Jensen, L.R.; Kihlström, L.; Förander, P.; Orrego, A.; Mathiesen, T. The Ki-67 Proliferation Index as a Marker of Time to Recurrence in Intracranial Meningioma. Neurosurgery 2020, 87, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Pettersson-Segerlind, J.; von Vogelsang, A.C.; Fletcher-Sandersjöö, A.; Tatter, C.; Mathiesen, T.; Edström, E.; Elmi-Terander, A. Health-Related Quality of Life and Return to Work after Surgery for Spinal Meningioma: A Population-Based Cohort Study. Cancers 2021, 13, 6371. [Google Scholar] [CrossRef] [PubMed]
- Lisi, L.; Ciotti, G.M.; Braun, D.; Kalinin, S.; Curro, D.; Dello Russo, C.; Coli, A.; Mangiola, A.; Anile, C.; Feinstein, D.L.; et al. Expression of iNOS, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci. Lett. 2017, 645, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, L.; Che, G.; Yu, N.; Dai, F.; You, Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 2010, 10, 112. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Rossi, M.L.; Cruz Sanchez, F.; Hughes, J.T.; Esiri, M.M.; Coakham, H.B. Immunocytochemical study of the cellular immune response in meningiomas. J. Clin. Pathol. 1988, 41, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Grund, S.; Schittenhelm, J.; Roser, F.; Tatagiba, M.; Mawrin, C.; Kim, Y.J.; Bornemann, A. The microglial/macrophagic response at the -brain border of invasive meningiomas. Neuropathol. Appl. Neurobiol. 2009, 35, 82–88. [Google Scholar] [CrossRef]
- Gadot, R.; Khan, A.B.; Patel, R.; Goethe, E.; Shetty, A.; Hadley, C.C.; Bayley, J.C.; Harmanci, A.S.; Klisch, T.; Yoshor, D.; et al. Predictors of postoperative seizure outcome in supratentorial meningioma. J. Neurosurg. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Waagemans, M.L.; van Nieuwenhuizen, D.; Dijkstra, M.; Wumkes, M.; Dirven, C.M.; Leenstra, S.; Reijneveld, J.C.; Klein, M.; Stalpers, L.J. Long-term impact of cognitive deficits and epilepsy on quality of life in patients with low-grade meningiomas. Neurosurgery 2011, 69, 72–79. [Google Scholar] [CrossRef]
- Lieu, A.S.; Howng, S.L. Intracranial meningiomas and epilepsy: Incidence, prognosis and influencing factors. Epilepsy Res. 2000, 38, 45–52. [Google Scholar] [CrossRef]
- Presta, I.; Guadagno, E.; Di Vito, A.; Malara, N.; Mignogna, C.; Maisano, D.; Donato, A.; Cardillo, G.; Del Basso De Caro, M.L.; Donato, G. Innate immunity may play a role in growth and relapse of chordoid meningioma. Int. J. Immunopathol. Pharmacol. 2017, 30, 429–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.J.; Reis, G.; Kohanbash, G.; Shrivastav, S.; Magill, S.T.; Molinaro, A.M.; McDermott, M.W.; Theodosopoulos, P.V.L.; Aghi, M.K.; Berger, M.S.; et al. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J. Neurooncol. 2016, 130, 543–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arasanz, H.; Gato-Cañas, M.; Zuazo, M.; Ibañez-Vea, M.; Breckpot, K.; Kochan, G.; Escors, D. PD1 signal transduction pathways in T cells. Oncotarget 2017, 8, 51936–51945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garzon-Muvdi, T.; Bailey, D.D.; Pernik, M.N.; Pan, E. Basis for Immunotherapy for Treatment of Meningiomas. Front. Neurol. 2020, 11, 945. [Google Scholar] [CrossRef]
- Ersoz, S.; Yilmaz, Z.S.; Eyuboglu, I.; Yazar, U. Xanthomatous Meningioma: A Case Report. Turk. Neurosurg. 2019, 29, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Ishida, M.; Fukami, T.; Nitta, N.; Iwai, M.; Yoshida, K.; Kagotani, A.; Nozaki, K.; Okabe, H. Xanthomatous meningioma: A case report with review of the literature. Int. J. Clin. Exp. Pathol. 2013, 6, 2242–2246. [Google Scholar]
- Liu, L.; Stone, J.; Hoffpauir, J.T.; Xiong, Z. Histiocytic meningioma: A distinctive subtype of meningioma? Intractable Rare Dis. Res. 2014, 3, 57–59. [Google Scholar] [CrossRef] [Green Version]







| Variable | Cranial (n = 418) | Spinal (n = 123) | p-Value |
|---|---|---|---|
| Sex | 0.09 | ||
| Female | 290 (69.4%) | 95 (77.2%) | |
| Male | 128 (30.6%) | 28 (22.8%) | |
| Age | 0.001 | ||
| Mean +/− SD | 60.7 +/− 13.6 | 65.6 +/− 12.4 | |
| Baseline KPS | 0.001 | ||
| Mean +/− SD | 89.8 +/− 11.5 | 82.1 +/− 11.0 | |
| BMI | 0.12 | ||
| Mean +/− SD | 27.3 +/− 5.9 | 28.3 +/− 5.1 | |
| Serum CRP | 0.04 | ||
| Mean +/− SD | 3.5 +/− 8.0 | 5.5 +/−9.8 | |
| Plasma fibrinogen | 0.003 | ||
| Mean +/− SD | 3.0 +/− 0.8 | 3.4 +/− 0.8 | |
| WHO grade | 0.005 | ||
| 1 | 344 (82.3%) | 114 (92.7%) | |
| 2 | 74 (17.7%) | 9 (7.3%) | |
| Mitotic count | 0.002 | ||
| Mean +/− SD | 1.9 +/− 2.8 | 1.1 +/− 1.2 | |
| MIB-1 index | 0.007 | ||
| Mean +/− SD | 5.4 +/− 3.0 | 4.3 +/− 2.4 | |
| CD68+ staining (n = 376) | 0.001 | ||
| Focal | 80 (27.8%) | 44 (50.0%) | |
| Diffuse | 208 (72.2%) | 44 (50.0%) | |
| CD68− staining (n = 165) | 130 (31.1%) | 35 (28.5%) | 0.27 |
| Variable | Non-Skull Base (n = 200) | Lateral Skull Base (n = 93) | Medial Skull Base (n = 125) | Spinal (n = 123) | p-Value |
|---|---|---|---|---|---|
| Sex | 0.07 | ||||
| Female | 136 (68.0%) | 60 (64.5%) | 95 (76.0%) | 95 (77.2%) | |
| Male | 64 (32.0%) | 33 (35.5%) | 30 (24.0%) | 28 (22.8%) | |
| Age | 0.001 | ||||
| Mean +/− SD | 62.1 +/− 13.6 | 59.6 +/− 13.2 | 59.5 +/− 13.7 | 65.6 +/− 12.4 | |
| Baseline KPS | 0.001 | ||||
| Mean +/− SD | 89.9 +/− 11.4 | 91.3 +/− 9.8 | 88.4 +/− 12.5 | 82.1 +/− 11.0 | |
| BMI | 0.18 | ||||
| Mean +/− SD | 27.6 +/− 6.1 | 26.2 +/− 4.8 | 27.4 +/− 6.2 | 28.3 +/− 5.1 | |
| Serum CRP | 0.03 | ||||
| Mean +/− SD | 4.3 +/− 10.9 | 2.5 +/− 3.1 | 2.9 +/− 3.8 | 5.5 +/− 9.8 | |
| WHO grade | 0.007 | ||||
| 1 | 157 (78.5%) | 79 (84.9%) | 108 (86.4%) | 114 (92.7%) | |
| 2 | 43 (21.5%) | 14 (15.1%) | 17 (13.6%) | 9 (7.3%) | |
| Mitotic count | 0.02 | ||||
| Mean +/− SD | 2.2 +/− 3.0 | 1.4 +/− 2.0 | 1.5 +/− 2.7 | 1.1 +/− 1.2 | |
| MIB-1 index | 0.001 | ||||
| Mean +/− SD | 6.2 +/− 3.5 | 4.7 +/− 2.1 | 4.6 +/− 2.1 | 4.3 +/− 2.4 | |
| CD68 staining (n = 376) | 0.001 | ||||
| Focal | 42 (25.0%) | 18 (32.7%) | 20 (27.5%) | 44 (50.0%) | |
| Diffuse | 111 (75.0%) | 37 (67.3%) | 60 (72.5%) | 44 (50.0%) |
| Variable | Mean +/− SD | Mean Difference | 95% CI of the Difference | p-Value |
|---|---|---|---|---|
| Non-skull base | 6.2 +/− 3.5 | 1.48 | 0.83–2.13 | 0.001 |
| vs. | ||||
| lateral skull base | 4.7 +/− 2.1 | |||
| Non-skull base | 6.2 +/− 3.5 | 1.57 | 0.96–2.18 | 0.001 |
| vs. | ||||
| medial skull base | 4.6 +/− 2.1 | |||
| Non-skull base | 6.2 +/− 3.5 | 1.85 | 1.20–2.50 | 0.001 |
| vs. | ||||
| spinal | 4.3 +/− 2.4 | |||
| lateral skull base | 4.7 +/− 2.1 | 0.09 | −0.65–0.47 | 0.76 |
| vs. | ||||
| medial skull base | 4.6 +/− 2.1 | |||
| lateral skull base | 4.7 +/− 2.1 | 0.37 | −0.25–0.99 | 0.24 |
| vs. | ||||
| spinal | 4.3 +/− 2.4 | |||
| medial skull base | 4.6 +/− 2.1 | 0.29 | −0.28–0.85 | 0.32 |
| vs. | ||||
| spinal | 4.3 +/− 2.4 |
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.15 | 0.54–2.49 | 0.70 | 1.05 | 0.48–2.32 | 0.90 |
| (>60 vs. ≤60) | ||||||
| Sex (male vs. female) | 2.00 | 0.91–4.39 | 0.08 | 2.44 | 1.06–5.64 | 0.04 |
| Karnofsky Performance Status (<90 vs. ≥90) | 2.00 | 0.94–4.26 | 0.07 | 2.37 | 1.05–5.33 | 0.04 |
| Location (Cranial vs. spinal) | 4.56 | 1.07–19.37 | 0.04 | 4.71 | 1.04–21.3 | 0.04 |
| Simpson grade (II vs. I) | 2.68 | 1.19–6.00 | 0.02 | 2.89 | 1.24–6.76 | 0.01 |
| WHO grade (2 vs. 1) | 4.95 | 2.31–10.62 | 0.001 | 4.08 | 1.84–9.05 | 0.001 |
| Variable | Cranial (n = 418) | Spinal (n = 123) | p-Value |
|---|---|---|---|
| Surgical site infections | 16 (3.8%) | 1 (0.8%) | 0.16 |
| Postoperative hemorrhage | 12 (2.8%) | 2 (1.6%) | 0.66 |
| Postoperative CSF fistula | 12 (2.8%) | 3 (2.4%) | 0.80 |
| New onset epilepsy | 24 (6.0%) | - | |
| Pulmonary embolism | 5 (1.2%) | 1 (0.8%) | 0.72 |
| Length of stay (in days) | |||
| Mean +/− SD | 11.0 +/− 8.1 | 13.1 +/− 12.7 | 0.08 |
| Baseline KPS | 0.001 | ||
| Mean +/− SD | 89.8 +/− 11.5 | 82.1 +/− 11.0 | |
| KPS at 3 months | 0.81 | ||
| Mean +/− SD | 88.7 +/− 17.9 | 88.1 +/− 10.0 | |
| KPS at 12 months | 0.54 | ||
| Mean +/− SD | 87.8 +/− 20.5 | 89.6 +/− 9.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wach, J.; Lampmann, T.; Güresir, Á.; Vatter, H.; Herrlinger, U.; Becker, A.; Cases-Cunillera, S.; Hölzel, M.; Toma, M.; Güresir, E. Proliferative Potential, and Inflammatory Tumor Microenvironment in Meningioma Correlate with Neurological Function at Presentation and Anatomical Location—From Convexity to Skull Base and Spine. Cancers 2022, 14, 1033. https://doi.org/10.3390/cancers14041033
Wach J, Lampmann T, Güresir Á, Vatter H, Herrlinger U, Becker A, Cases-Cunillera S, Hölzel M, Toma M, Güresir E. Proliferative Potential, and Inflammatory Tumor Microenvironment in Meningioma Correlate with Neurological Function at Presentation and Anatomical Location—From Convexity to Skull Base and Spine. Cancers. 2022; 14(4):1033. https://doi.org/10.3390/cancers14041033
Chicago/Turabian StyleWach, Johannes, Tim Lampmann, Ági Güresir, Hartmut Vatter, Ulrich Herrlinger, Albert Becker, Silvia Cases-Cunillera, Michael Hölzel, Marieta Toma, and Erdem Güresir. 2022. "Proliferative Potential, and Inflammatory Tumor Microenvironment in Meningioma Correlate with Neurological Function at Presentation and Anatomical Location—From Convexity to Skull Base and Spine" Cancers 14, no. 4: 1033. https://doi.org/10.3390/cancers14041033
APA StyleWach, J., Lampmann, T., Güresir, Á., Vatter, H., Herrlinger, U., Becker, A., Cases-Cunillera, S., Hölzel, M., Toma, M., & Güresir, E. (2022). Proliferative Potential, and Inflammatory Tumor Microenvironment in Meningioma Correlate with Neurological Function at Presentation and Anatomical Location—From Convexity to Skull Base and Spine. Cancers, 14(4), 1033. https://doi.org/10.3390/cancers14041033