Recent Developments of Circulating Tumor Cell Analysis for Monitoring Cutaneous Melanoma Patients
Abstract
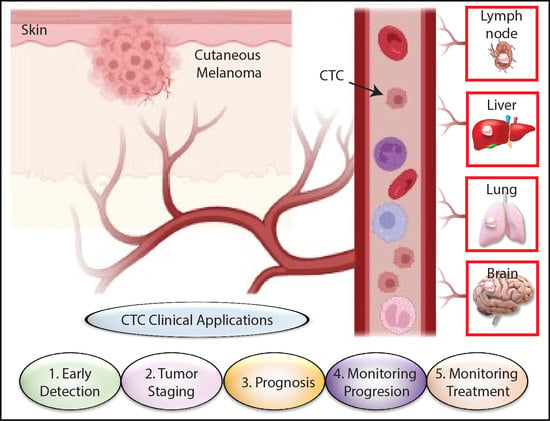
:Simple Summary
Abstract
1. Introduction
2. Literature Review
3. Detection of Melanoma CTCs Using Multi-Marker RT-PCR Assays
4. Multicenter Trials on CTC Detection for Melanoma Patients
5. CTC Detection in Association with Treatment Response
6. CTC Enrichment and Detection Using CELLSEARCH® System
7. CTC and Genomic Biomarkers
8. Other Prognostic Biomarker for Cutaneous Melanoma Patients
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, S.K.; Hoon, D.S. Liquid biopsy utility for the surveillance of cutaneous malignant melanoma patients. Mol. Oncol. 2016, 10, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Testori, A.A.E.; Chiellino, S.; van Akkooi, A.C.J. Adjuvant Therapy for Melanoma: Past, Current, and Future Developments. Cancers 2020, 12, 1994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bustos, M.A.; Shoji, Y.; Ramos, R.I.; Iida, Y.; Gentry, R.; Takeshima, T.L.; Hoon, D.S.B. Acetylated DNMT1 Downregulation and Related Regulatory Factors Influence Metastatic Melanoma Patients Survival. Cancers 2021, 13, 4691. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Rebecca, V.W.; Somasundaram, R.; Herlyn, M. Pre-clinical modeling of cutaneous melanoma. Nat. Commun. 2020, 11, 2858. [Google Scholar] [CrossRef]
- Zhang, X.; Bustos, M.A.; Gross, R.; Ramos, R.I.; Takeshima, T.L.; Mills, G.B.; Yu, Q.; Hoon, D.S.B. Interleukin enhancer-binding factor 2 promotes cell proliferation and DNA damage response in metastatic melanoma. Clin. Transl. Med. 2021, 11, e608. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, R.; Koyanagi, K.; Narita, N.; Kuo, C.; Hoon, D.S. Prognostic molecular biomarkers for cutaneous malignant melanoma. J. Surg. Oncol. 2011, 104, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, E.S.; Chong, K.K.; Huynh, K.T.; Tanaka, R.; Hoon, D.S. Epigenetic biomarkers in skin cancer. Cancer Lett. 2014, 342, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Keller, L.; Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 2019, 19, 553–567. [Google Scholar] [CrossRef] [Green Version]
- Au, S.H.; Edd, J.; Stoddard, A.E.; Wong, K.H.K.; Fachin, F.; Maheswaran, S.; Haber, D.A.; Stott, S.L.; Kapur, R.; Toner, M. Microfluidic Isolation of Circulating Tumor Cell Clusters by Size and Asymmetry. Sci. Rep. 2017, 7, 2433. [Google Scholar] [CrossRef]
- Hong, Y.; Fang, F.; Zhang, Q. Circulating tumor cell clusters: What we know and what we expect (Review). Int. J. Oncol. 2016, 49, 2206–2216. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, A.; Koppaka, D.; Anand, A.; Deb, B.; Grenci, G.; Viasnoff, V.; Thompson, E.W.; Gowda, H.; Bhat, R.; Rangarajan, A.; et al. Circulating Tumor Cell cluster phenotype allows monitoring response to treatment and predicts survival. Sci. Rep. 2019, 9, 7933. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, K.J.; MacDonald, I.C.; Schmidt, E.E.; Kerkvliet, N.; Morris, V.L.; Chambers, A.F.; Groom, A.C. Multistep nature of metastatic inefficiency: Dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 1998, 153, 865–873. [Google Scholar] [CrossRef]
- De Souza, L.M.; Robertson, B.M.; Robertson, G.P. Future of circulating tumor cells in the melanoma clinical and research laboratory settings. Cancer Lett. 2017, 392, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Khoja, L.; Lorigan, P.; Dive, C.; Keilholz, U.; Fusi, A. Circulating tumour cells as tumour biomarkers in melanoma: Detection methods and clinical relevance. Ann. Oncol. 2015, 26, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Marsavela, G.; Aya-Bonilla, C.A.; Warkiani, M.E.; Gray, E.S.; Ziman, M. Melanoma circulating tumor cells: Benefits and challenges required for clinical application. Cancer Lett. 2018, 424, 1–8. [Google Scholar] [CrossRef]
- Lianidou, E.; Hoon, D. Circulating tumor cells and circulating tumor DNA. In Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th ed.; Nader, R., Horrath, A., Wittwer, C., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 1111–1144. [Google Scholar]
- Paterlini-Brechot, P.; Benali, N.L. Circulating tumor cells (CTC) detection: Clinical impact and future directions. Cancer Lett. 2007, 253, 180–204. [Google Scholar] [CrossRef]
- Michaelson, J.S.; Cheongsiatmoy, J.A.; Dewey, F.; Silverstein, M.J.; Sgroi, D.; Smith, B.; Tanabe, K.K. Spread of human cancer cells occurs with probabilities indicative of a nongenetic mechanism. Br. J. Cancer 2005, 93, 1244–1249. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.C.; Teng, P.C.; Chen, P.J.; Posadas, E.; Tseng, H.R.; Lu, S.C.; Yang, J.D. Detection of circulating tumor cells and their implications as a novel biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatology 2020, 73, 422–436. [Google Scholar] [CrossRef]
- Habli, Z.; AlChamaa, W.; Saab, R.; Kadara, H.; Khraiche, M.L. Circulating Tumor Cell Detection Technologies and Clinical Utility: Challenges and Opportunities. Cancers 2020, 12, 1930. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Hoon, D.; Ambrosi, A.; Nitti, D.; Rossi, C.R. The prognostic value of circulating tumor cells in patients with melanoma: A systematic review and meta-analysis. Clin. Cancer Res. 2006, 12, 4605–4613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulmer, A.; Schmidt-Kittler, O.; Fischer, J.; Ellwanger, U.; Rassner, G.; Riethmuller, G.; Fierlbeck, G.; Klein, C.A. Immunomagnetic enrichment, genomic characterization, and prognostic impact of circulating melanoma cells. Clin. Cancer Res. 2004, 10, 531–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, M.M.; Ramani, V.C.; Jeffrey, S.S. Circulating tumor cell technologies. Mol. Oncol. 2016, 10, 374–394. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, G.; Satriano, S.M.; Budroni, M.; Cossu, A.; Tanda, F.; Canzanella, S.; Caraco, C.; Simeone, E.; Daponte, A.; Mozzillo, N.; et al. Serial detection of circulating tumour cells by reverse transcriptase-polymerase chain reaction assays is a marker for poor outcome in patients with malignant melanoma. BMC Cancer 2006, 6, 266. [Google Scholar] [CrossRef] [Green Version]
- Scoggins, C.R.; Ross, M.I.; Reintgen, D.S.; Noyes, R.D.; Goydos, J.S.; Beitsch, P.D.; Urist, M.M.; Ariyan, S.; Davidson, B.S.; Sussman, J.J.; et al. Prospective multi-institutional study of reverse transcriptase polymerase chain reaction for molecular staging of melanoma. J. Clin. Oncol. 2006, 24, 2849–2857. [Google Scholar] [CrossRef]
- Koyanagi, K.; Mori, T.; O’Day, S.J.; Martinez, S.R.; Wang, H.J.; Hoon, D.S. Association of circulating tumor cells with serum tumor-related methylated DNA in peripheral blood of melanoma patients. Cancer Res. 2006, 66, 6111–6117. [Google Scholar] [CrossRef] [Green Version]
- Koyanagi, K.; O’Day, S.J.; Gonzalez, R.; Lewis, K.; Robinson, W.A.; Amatruda, T.T.; Kuo, C.; Wang, H.J.; Milford, R.; Morton, D.L.; et al. Microphthalmia transcription factor as a molecular marker for circulating tumor cell detection in blood of melanoma patients. Clin. Cancer Res. 2006, 12, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, E.; Prados, J.; Marchal, J.A.; Boulaiz, H.; Martinez, A.; Rodriguez-Serrano, F.; Caba, O.; Serrano, S.; Aranega, A. Prognostic value of RT-PCR tyrosinase detection in peripheral blood of melanoma patients. Dis. Markers 2006, 22, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Quaglino, P.; Osella-Abate, S.; Cappello, N.; Ortoncelli, M.; Nardo, T.; Fierro, M.T.; Cavallo, F.; Savoia, P.; Bernengo, M.G. Prognostic relevance of baseline and sequential peripheral blood tyrosinase expression in 200 consecutive advanced metastatic melanoma patients. Melanoma Res. 2007, 17, 75–82. [Google Scholar] [CrossRef]
- Visus, C.; Andres, R.; Mayordomo, J.I.; Martinez-Lorenzo, M.J.; Murillo, L.; Saez-Gutierrez, B.; Diestre, C.; Marcos, I.; Astier, P.; Godino, J.; et al. Prognostic role of circulating melanoma cells detected by reverse transcriptase-polymerase chain reaction for tyrosinase mRNA in patients with melanoma. Melanoma Res. 2007, 17, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Arenberger, P.; Arenbergerova, M.; Gkalpakiotis, S.; Lippert, J.; Stribrna, J.; Kremen, J. Multimarker real-time reverse transcription-PCR for quantitative detection of melanoma-associated antigens: A novel possible staging method. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Nowecki, Z.I.; Kulik, J.; Ruka, W.; Siedlecki, J.A. Molecular staging by multimarker reverse transcriptase-polymerase chain reaction assay of lymphatic drainage and blood from melanoma patients after lymph node dissection. Melanoma Res. 2008, 18, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Fusi, A.; Collette, S.; Busse, A.; Suciu, S.; Rietz, A.; Santinami, M.; Kruit, W.H.; Testori, A.; Punt, C.J.; Dalgleish, A.G.; et al. Circulating melanoma cells and distant metastasis-free survival in stage III melanoma patients with or without adjuvant interferon treatment (EORTC 18991 side study). Eur. J. Cancer 2009, 45, 3189–3197. [Google Scholar] [CrossRef]
- Gkalpakiotis, S.; Arenberger, P.; Kremen, J.; Arenbergerova, M. Quantitative detection of melanoma-associated antigens by multimarker real-time RT-PCR for molecular staging: Results of a 5 years study. Exp. Dermatol. 2010, 19, 994–999. [Google Scholar] [CrossRef]
- Koyanagi, K.; O’Day, S.J.; Boasberg, P.; Atkins, M.B.; Wang, H.J.; Gonzalez, R.; Lewis, K.; Thompson, J.A.; Anderson, C.M.; Lutzky, J.; et al. Serial monitoring of circulating tumor cells predicts outcome of induction biochemotherapy plus maintenance biotherapy for metastatic melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 2402–2408. [Google Scholar] [CrossRef] [Green Version]
- Samija, I.; Lukac, J.; Maric-Brozic, J.; Buljan, M.; Alajbeg, I.; Kovacevic, D.; Situm, M.; Kusic, Z. Prognostic value of microphthalmia-associated transcription factor and tyrosinase as markers for circulating tumor cells detection in patients with melanoma. Melanoma Res. 2010, 20, 293–302. [Google Scholar] [CrossRef]
- Rao, C.; Bui, T.; Connelly, M.; Doyle, G.; Karydis, I.; Middleton, M.R.; Clack, G.; Malone, M.; Coumans, F.A.; Terstappen, L.W. Circulating melanoma cells and survival in metastatic melanoma. Int. J. Oncol. 2011, 38, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Hoshimoto, S.; Faries, M.B.; Morton, D.L.; Shingai, T.; Kuo, C.; Wang, H.J.; Elashoff, R.; Mozzillo, N.; Kelley, M.C.; Thompson, J.F.; et al. Assessment of prognostic circulating tumor cells in a phase III trial of adjuvant immunotherapy after complete resection of stage IV melanoma. Ann. Surg. 2012, 255, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Hoshimoto, S.; Shingai, T.; Morton, D.L.; Kuo, C.; Faries, M.B.; Chong, K.; Elashoff, D.; Wang, H.J.; Elashoff, R.M.; Hoon, D.S. Association between circulating tumor cells and prognosis in patients with stage III melanoma with sentinel lymph node metastasis in a phase III international multicenter trial. J. Clin. Oncol. 2012, 30, 3819–3826. [Google Scholar] [CrossRef] [Green Version]
- Khoja, L.; Lorigan, P.; Zhou, C.; Lancashire, M.; Booth, J.; Cummings, J.; Califano, R.; Clack, G.; Hughes, A.; Dive, C. Biomarker utility of circulating tumor cells in metastatic cutaneous melanoma. J. Invest. Dermatol. 2013, 133, 1582–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, A.L.; Millward, M.; Pearce, R.; Lee, M.; Frank, M.H.; Ireland, A.; Monshizadeh, L.; Rai, T.; Heenan, P.; Medic, S.; et al. Markers of circulating tumour cells in the peripheral blood of patients with melanoma correlate with disease recurrence and progression. Br. J. Dermatol. 2013, 168, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.S.; Reid, A.L.; Bowyer, S.; Calapre, L.; Siew, K.; Pearce, R.; Cowell, L.; Frank, M.H.; Millward, M.; Ziman, M. Circulating Melanoma Cell Subpopulations: Their Heterogeneity and Differential Responses to Treatment. J. Invest. Dermatol. 2015, 135, 2040–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roland, C.L.; Ross, M.I.; Hall, C.S.; Laubacher, B.; Upshaw, J.; Anderson, A.E.; Lucci, A. Detection of circulating melanoma cells in the blood of melanoma patients: A preliminary study. Melanoma Res. 2015, 25, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Fu, W.; Zhang, W.; Li, P. High Number of Circulating Tumor Cells Predicts Poor Survival of Cutaneous Melanoma Patients in China. Med. Sci. Monit. 2018, 24, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.S.; Ross, M.; Bowman Bauldry, J.B.; Upshaw, J.; Karhade, M.G.; Royal, R.; Patel, S.; Lucci, A. Circulating Tumor Cells in Stage IV Melanoma Patients. J. Am. Coll. Surg. 2018, 227, 116–124. [Google Scholar] [CrossRef]
- Lucci, A.; Hall, C.S.; Patel, S.P.; Narendran, B.; Bauldry, J.B.; Royal, R.E.; Karhade, M.; Upshaw, J.R.; Wargo, J.A.; Glitza, I.C.; et al. Circulating Tumor Cells and Early Relapse in Node-positive Melanoma. Clin. Cancer Res. 2020, 26, 1886–1895. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chang, S.C.; Lam, S.; Irene Ramos, R.; Tran, K.; Ohe, S.; Salomon, M.P.; Bhagat, A.A.S.; Teck Lim, C.; Fischer, T.D.; et al. Prospective Molecular Profiling of Circulating Tumor Cells from Patients with Melanoma Receiving Combinatorial Immunotherapy. Clin. Chem. 2020, 66, 169–177. [Google Scholar] [CrossRef]
- Hoon, D.S.; Wang, Y.; Dale, P.S.; Conrad, A.J.; Schmid, P.; Garrison, D.; Kuo, C.; Foshag, L.J.; Nizze, A.J.; Morton, D.L. Detection of occult melanoma cells in blood with a multiple-marker polymerase chain reaction assay. J. Clin. Oncol. 1995, 13, 2109–2116. [Google Scholar] [CrossRef]
- Van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991, 254, 1643–1647. [Google Scholar] [CrossRef]
- De Plaen, E.; Arden, K.; Traversari, C.; Gaforio, J.J.; Szikora, J.P.; De Smet, C.; Brasseur, F.; van der Bruggen, P.; Lethé, B.; Lurquin, C.; et al. Structure, chromosomal localization, and expression of 12 genes of the MAGE family. Immunogenetics 1994, 40, 360–369. [Google Scholar] [CrossRef]
- Güre, A.O.; Stockert, E.; Arden, K.C.; Boyer, A.D.; Viars, C.S.; Scanlan, M.J.; Old, L.J.; Chen, Y.T. CT10: A new cancer-testis (CT) antigen homologous to CT7 and the MAGE family, identified by representational-difference analysis. Int. J. Cancer 2000, 85, 726–732. [Google Scholar] [CrossRef]
- Brasseur, F.; Rimoldi, D.; Liénard, D.; Lethé, B.; Carrel, S.; Arienti, F.; Suter, L.; Vanwijck, R.; Bourlond, A.; Humblet, Y.; et al. Expression of MAGE genes in primary and metastatic cutaneous melanoma. Int. J. Cancer 1995, 63, 375–380. [Google Scholar] [CrossRef]
- Miyashiro, I.; Kuo, C.; Huynh, K.; Iida, A.; Morton, D.; Bilchik, A.; Giuliano, A.; Hoon, D.S. Molecular strategy for detecting metastatic cancers with use of multiple tumor-specific MAGE-A genes. Clin. Chem. 2001, 47, 505–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggermont, A.M.; Suciu, S.; Santinami, M.; Testori, A.; Kruit, W.H.; Marsden, J.; Punt, C.J.; Sales, F.; Gore, M.; Mackie, R.; et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: Final results of EORTC 18991, a randomised phase III trial. Lancet 2008, 372, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Morton, D.L.; Mozzillo, N.; Thompson, J.F.; Kelley, M.C.; Faries, M.; Wagner, J.; Schneebaum, S.; Schuchter, L.; Gammon, G.; Elashoff, R. An international, randomized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. J. Clin. Oncol. 2007, 25, 8508. [Google Scholar] [CrossRef]
- Keung, E.Z.; Gershenwald, J.E. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: Implications for melanoma treatment and care. Expert Rev. Anticancer Ther. 2018, 18, 775–784. [Google Scholar] [CrossRef]
- Kuo, C.T.; Bostick, P.J.; Irie, R.F.; Morton, D.L.; Conrad, A.J.; Hoon, D.S. Assessment of messenger RNA of beta 1—>4-N-acetylgalactosaminyl-transferase as a molecular marker for metastatic melanoma. Clin. Cancer Res. 1998, 4, 411–418. [Google Scholar] [PubMed]
- Ramos, R.I.; Bustos, M.A.; Wu, J.; Jones, P.; Chang, S.C.; Kiyohara, E.; Tran, K.; Zhang, X.; Stern, S.L.; Izraely, S.; et al. Upregulation of cell surface GD3 ganglioside phenotype is associated with human melanoma brain metastasis. Mol. Oncol. 2020, 14, 1760–1778. [Google Scholar] [CrossRef] [PubMed]
- Groux-Degroote, S.; Guérardel, Y.; Delannoy, P. Gangliosides: Structures, Biosynthesis, Analysis, and Roles in Cancer. Chembiochem 2017, 18, 1146–1154. [Google Scholar] [CrossRef] [Green Version]
- O’Day, S.J.; Atkins, M.B.; Boasberg, P.; Wang, H.J.; Thompson, J.A.; Anderson, C.M.; Gonzalez, R.; Lutzky, J.; Amatruda, T.; Hersh, E.M.; et al. Phase II multicenter trial of maintenance biotherapy after induction concurrent Biochemotherapy for patients with metastatic melanoma. J. Clin. Oncol. 2009, 27, 6207–6212. [Google Scholar] [CrossRef]
- Rapanotti, M.C.; Campione, E.; Spallone, G.; Orlandi, A.; Bernardini, S.; Bianchi, L. Minimal residual disease in melanoma: Circulating melanoma cells and predictive role of MCAM/MUC18/MelCAM/CD146. Cell Death Discov. 2017, 3, 17005. [Google Scholar] [CrossRef] [Green Version]
- Hoon, D.S.; Spugnardi, M.; Kuo, C.; Huang, S.K.; Morton, D.L.; Taback, B. Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene 2004, 23, 4014–4022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.L.; Lim, T.H.; Lim, T.; Tan, D.S.; Chua, Y.W.; Ang, M.K.; Pang, B.; Lim, C.T.; Takano, A.; Lim, A.S.; et al. Concordance of anaplastic lymphoma kinase (ALK) gene rearrangements between circulating tumor cells and tumor in non-small cell lung cancer. Oncotarget 2016, 7, 23251–23262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, E.; Zamarchi, R. Single-Cell Analysis of Circulating Tumor Cells: How Far Have We Come in the-Omics Era? Front. Genet. 2019, 10, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustos, M.A.; Tran, K.D.; Rahimzadeh, N.; Gross, R.; Lin, S.Y.; Shoji, Y.; Murakami, T.; Boley, C.L.; Tran, L.T.; Cole, H.; et al. Integrated Assessment of Circulating Cell-Free MicroRNA Signatures in Plasma of Patients with Melanoma Brain Metastasis. Cancers 2020, 12, 1692. [Google Scholar] [CrossRef]
- Bollard, S.M.; Casalou, C.; Goh, C.Y.; Tobin, D.J.; Kelly, P.; McCann, A.; Potter, S.M. Circulating Melanoma-Derived Extracellular Vesicles: Impact on Melanoma Diagnosis, Progression Monitoring, and Treatment Response. Pharmaceuticals 2020, 13, 475. [Google Scholar] [CrossRef]
- Hinestrosa, J.P.; Searson, D.J.; Lewis, J.M.; Kinana, A.; Perrera, O.; Dobrovolskaia, I.; Tran, K.; Turner, R.; Balcer, H.I.; Clark, I.; et al. Simultaneous Isolation of Circulating Nucleic Acids and EV-Associated Protein Biomarkers From Unprocessed Plasma Using an AC Electrokinetics-Based Platform. Front. Bioeng. Biotechnol. 2020, 8, 581157. [Google Scholar] [CrossRef]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A landscape of driver mutations in melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Said, R.; Guibert, N.; Oxnard, G.R.; Tsimberidou, A.M. Circulating tumor DNA analysis in the era of precision oncology. Oncotarget 2020, 11, 188–211. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, M.; O’Day, S.J.; Kitago, M.; Amersi, F.; Kuo, C.; Kim, J.; Wang, H.J.; Hoon, D.S. Utility of circulating B-RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clin. Cancer Res. 2007, 13, 2068–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascierto, P.A.; Minor, D.; Ribas, A.; Lebbe, C.; O’Hagan, A.; Arya, N.; Guckert, M.; Schadendorf, D.; Kefford, R.F.; Grob, J.J.; et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J. Clin. Oncol. 2013, 31, 3205–3211. [Google Scholar] [CrossRef]
- Hauschild, A.; Grob, J.J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H., Jr.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- Long, G.V.; Trefzer, U.; Davies, M.A.; Kefford, R.F.; Ascierto, P.A.; Chapman, P.B.; Puzanov, I.; Hauschild, A.; Robert, C.; Algazi, A.; et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 1087–1095. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Santiago-Walker, A.; Gagnon, R.; Mazumdar, J.; Casey, M.; Long, G.V.; Schadendorf, D.; Flaherty, K.; Kefford, R.; Hauschild, A.; Hwu, P.; et al. Correlation of BRAF Mutation Status in Circulating-Free DNA and Tumor and Association with Clinical Outcome across Four BRAFi and MEKi Clinical Trials. Clin. Cancer Res. 2016, 22, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Syeda, M.M.; Wiggins, J.M.; Corless, B.C.; Long, G.V.; Flaherty, K.T.; Schadendorf, D.; Nathan, P.D.; Robert, C.; Ribas, A.; Davies, M.A.; et al. Circulating tumour DNA in patients with advanced melanoma treated with dabrafenib or dabrafenib plus trametinib: A clinical validation study. Lancet Oncol. 2021, 22, 370–380. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.J.; Flaherty, K.T.; Arance, A.; Chiarion-Sileni, V.; Thomas, L.; Lesimple, T.; Mortier, L.; et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): A multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef]
- Lowes, L.E.; Bratman, S.V.; Dittamore, R.; Done, S.; Kelley, S.O.; Mai, S.; Morin, R.D.; Wyatt, A.W.; Allan, A.L. Circulating Tumor Cells (CTC) and Cell-Free DNA (cfDNA) Workshop 2016: Scientific Opportunities and Logistics for Cancer Clinical Trial Incorporation. Int. J. Mol. Sci. 2016, 17, 1505. [Google Scholar] [CrossRef] [Green Version]
- Beaver, J.A.; Jelovac, D.; Balukrishna, S.; Cochran, R.; Croessmann, S.; Zabransky, D.J.; Wong, H.Y.; Toro, P.V.; Cidado, J.; Blair, B.G.; et al. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin. Cancer Res. 2014, 20, 2643–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frenel, J.S.; Carreira, S.; Goodall, J.; Roda, D.; Perez-Lopez, R.; Tunariu, N.; Riisnaes, R.; Miranda, S.; Figueiredo, I.; Nava-Rodrigues, D.; et al. Serial Next-Generation Sequencing of Circulating Cell-Free DNA Evaluating Tumor Clone Response To Molecularly Targeted Drug Administration. Clin. Cancer Res. 2015, 21, 4586–4596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.; Black, J.R.M.; Reading, J.L.; Litchfield, K.; Turajlic, S.; McGranahan, N.; Jamal-Hanjani, M.; Swanton, C. Tracking Cancer Evolution through the Disease Course. Cancer Discov. 2021, 11, 916–932. [Google Scholar] [CrossRef]
- Lin, S.Y.; Huang, S.K.; Huynh, K.T.; Salomon, M.P.; Chang, S.C.; Marzese, D.M.; Lanman, R.B.; Talasaz, A.; Hoon, D.S.B. Multiplex Gene Profiling of Cell-Free DNA in Patients with Metastatic Melanoma for Monitoring Disease. JCO Precis. Oncol. 2018, 2, 1–30. [Google Scholar] [CrossRef]
- Marsavela, G.; McEvoy, A.C.; Pereira, M.R.; Reid, A.L.; Al-Ogaili, Z.; Warburton, L.; Khattak, M.A.; Abed, A.; Meniawy, T.M.; Millward, M.; et al. Detection of clinical progression through plasma ctDNA in metastatic melanoma patients: A comparison to radiological progression. Br. J. Cancer 2021, 126, 401–408. [Google Scholar] [CrossRef]
- Aya-Bonilla, C.A.; Morici, M.; Hong, X.; McEvoy, A.C.; Sullivan, R.J.; Freeman, J.; Calapre, L.; Khattak, M.A.; Meniawy, T.; Millward, M.; et al. Detection and prognostic role of heterogeneous populations of melanoma circulating tumour cells. Br. J. Cancer 2020, 122, 1059–1067. [Google Scholar] [CrossRef]

| Author | Year | Multicenter | N | Blood Draw | CTC Enrichment/ Detection | CTC Markers | AJCC Stage | Outcomes Related to CTC Detection |
|---|---|---|---|---|---|---|---|---|
| G Palmieri et al. [26] | 2006 | No | 149 | Multiple | MM PCR | MELTF, MLANA, TYR | I–III | Recurrence, Survival (DFS, OS) |
| CR Scoggins et al. [27] | 2006 | Yes | 820 | Multiple | MM PCR | MAGEA3, MLANA, PMEL, TYR | I, II | Survival (DDFS, DFS) |
| K Koyanagi et al. [28] | 2006 | No | 50 | Single | MM PCR | B4GALNT1, MAGEA3, MLANA | IV | Survival (OS, PFS), Treatment response |
| K Koyanagi et al. [29] | 2006 | No | 148 | Multiple | SM PCR | MITF | I–IV | AJCC Stage, Survival (OS, RFS) |
| E Carrillo et al. [30] | 2006 | No | 58 | Single | SM PCR | TYR | I-IV | AJCC Stage, Recurrence, Survival (OS) |
| P Quaglino et al. [31] | 2007 | No | 200 | Multiple | SM PCR | TYR | IV | Progression, Survival (OS, PFS), Treatment response |
| C Visús et al. [32] | 2007 | No | 114 | Single | SM PCR | TYR | I–IV | AJCC Stage, Survival (DFS, OS, PFS) |
| P Arenberger et al. [33] | 2008 | No | 65 | Multiple | MM PCR | MLANA, PMEL, MAGEA3, MIA, TYR | II–III | Progression |
| P Rutkowski et al. [34] | 2008 | No | 107 | Single | MM PCR | MLANA, TYR, uMAGE | III | None |
| A Fusi et al. [35] | 2009 | Yes | 299 | Multiple | MM PCR | MLANA, TYR | III | Progression |
| S Gkalpakiotis et al. [36] | 2010 | No | 65 | Multiple | MM PCR | MAGEA3, MIA, MLANA, PMEL, TYR | II, III | Progression, Survival (DFS) |
| K Koyanagi et al. [37] | 2010 | Yes | 87 | Multiple | MM PCR | B4GALNT1, MAGEA3, MITF, MLANA, PAX3, | IV | Progression, Survival (OS), Treatment response |
| I Samija et al. [38] | 2010 | No | 201 | Single | MM PCR | MITF, TYR | I-IV | Survival (OS, PFS) |
| C Rao et al. [39] | 2011 | No | 44 | Single | CELLSEARCH® | CD146, HMW-MAA | III, IV | Survival (OS) |
| S Hoshimoto et al. [40] | 2012 | Yes | 244 | Multiple | MM PCR | MAGEA3, MLANA, PAX3 | IV | Survival (DFS, OS) |
| S Hoshimoto et al. [41] | 2012 | Yes | 331 | Single | MM PCR | B4GALNT1, MAGEA3, MLANA | III | Survival (DDFS, DSS, RFS) |
| L Khoja et al. [42] | 2013 | No | 101 | Multiple | CELLSEARCH® | CD146, HMW-MAA | IV | Survival (OS) |
| AL Reid et al. [43] | 2013 | No | 230 | Multiple | MM PCR | ABCB5, MCAM, MLANA, PAX3, TGFB2, PAX3 | 0–IV | Recurrence, Treatment response |
| ES Gray et al. [44] | 2015 | No | 56 | Multiple | Flow cytometry | ABCB5, CD146, CD271, HMW-MAA, RANK | I–IV | Survival (PFS) |
| CL Roland et al. [45] | 2015 | No | 89 | Single | CELLSEARCH® | CD146, HMW-MAA | I–IV | AJCC Stage |
| J Li et al. [46] | 2018 | No | 100 | Multiple | CELLSEARCH® | CD146, HMW-MAA | I–IV | AJCC Stage, Survival (OS, PFS, DSS) |
| CS Hall et al. [47] | 2018 | No | 93 | Single | CELLSEARCH® | CD146, HMW-MAA | IV | Survival (PFS) |
| A Lucci et al. [48] | 2020 | No | 243 | Single | CELLSEARCH® | CD146, HMW-MAA | III | Survival (RFS) |
| SY Lin et al. [49] | 2020 | No | 75 | Multiple | ClearCell® FX/MM PCR | B4GALNT1, MAGEA3, MLANA, PAX3, TRP-AGG2-6 | III, IV | Survival (DFS, OS), Treatment response |
| Variables | N | |
|---|---|---|
| Study design | Single-center | 19 |
| Multicenter | 5 | |
| Disease status | Localized | 1 |
| Metastatic | 12 | |
| Both | 11 | |
| Blood draw | Single | 10 |
| Multiple | 14 | |
| CTC enrichment | CELLSEARCH® | 6 |
| ClearCell® FX | 1 | |
| None | 17 | |
| CTC detection | Multi-marker RT-PCR | 13 |
| Single-marker RT-PCR | 4 | |
| CellSearch | 6 | |
| Flow cytometry | 1 | |
| RT-PCR mRNA markers | MLANA | 12 |
| TYR | 10 | |
| MAGE | 9 | |
| PAX3 | 4 | |
| PMEL | 3 | |
| MITF | 3 | |
| MIA | 2 | |
| B4GALNT1 | 2 | |
| Outcomes | Survival | 19 |
| AJCC Stage | 5 | |
| Progression | 5 | |
| Treatment response | 5 | |
| Recurrence | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoji, Y.; Bustos, M.A.; Gross, R.; Hoon, D.S.B. Recent Developments of Circulating Tumor Cell Analysis for Monitoring Cutaneous Melanoma Patients. Cancers 2022, 14, 859. https://doi.org/10.3390/cancers14040859
Shoji Y, Bustos MA, Gross R, Hoon DSB. Recent Developments of Circulating Tumor Cell Analysis for Monitoring Cutaneous Melanoma Patients. Cancers. 2022; 14(4):859. https://doi.org/10.3390/cancers14040859
Chicago/Turabian StyleShoji, Yoshiaki, Matias A. Bustos, Rebecca Gross, and Dave S. B. Hoon. 2022. "Recent Developments of Circulating Tumor Cell Analysis for Monitoring Cutaneous Melanoma Patients" Cancers 14, no. 4: 859. https://doi.org/10.3390/cancers14040859
APA StyleShoji, Y., Bustos, M. A., Gross, R., & Hoon, D. S. B. (2022). Recent Developments of Circulating Tumor Cell Analysis for Monitoring Cutaneous Melanoma Patients. Cancers, 14(4), 859. https://doi.org/10.3390/cancers14040859