Proton Radiation Therapy for Nasopharyngeal Cancer Patients: Dosimetric and NTCP Evaluation Supporting Clinical Decision
Abstract
:Simple Summary
Abstract
1. Introduction
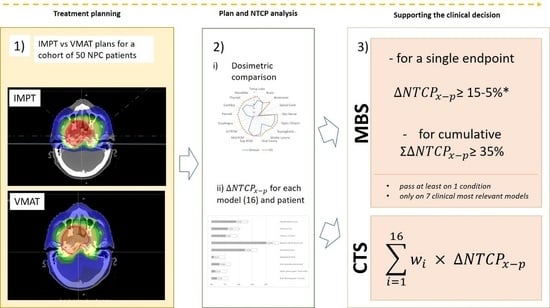
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, L.L.; Chen, W.Q.; Xue, W.Q.; He, Y.Q.; Zheng, R.S.; Zeng, Y.X.; Jia, W.H. Global Trends in Incidence and Mortality of Nasopharyngeal Carcinoma. Cancer Lett. 2016, 374, 22–30. [Google Scholar] [CrossRef]
- Bossi, P.; Trama, A.; Bernasconi, A.; Grisanti, S.; Mohamad, I.; Linares Galiana, I.; Ozyar, E.; Franco, P.; Vecchio, S.; Bonomo, P.; et al. Nasopharyngeal Cancer in Non-Endemic Areas: Impact of Treatment Intensity within a Large Retrospective Multicenter Cohort. Eur. J. Cancer 2021, 159, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.C.; Grégoire, V.; Lefebvre, J.L.; Licitra, L.; Hui, E.P.; Leung, S.F.; Felip, E. Nasopharyngeal Cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for Diagnosis, Treatment Andfollow-Up. Ann. Oncol. 2012, 23, vii83–vii85. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Han, F.; Xiao, W.; Xiang, Y.; Lu, L.; Deng, X.; Cui, N.; Zhao, C. Analysis of Late Toxicity in Nasopharyngeal Carcinoma Patients Treated with Intensity Modulated Radiation Therapy. Radiat. Oncol. 2015, 10, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.-L.; Chien, C.-Y.; Tsai, W.-L.; Liao, K.-C.; Chou, S.-Y.; Lin, H.-C.; Luo, S.D.; Lee, T.-F.; Lee, C.-H.; Fang, F.-M. Long-Term Late Toxicities and Quality of Life for Survivors of Nasopharyngeal Carcinoma Treated with Intensity-Modulated Radiotherapy versus Non–Intensity-Modulated Radiotherapy. Head Neck 2016, 38, E1026–E1032. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.R.; Liu, Y.T.; Jiang, N.; Fan, Y.X.; Wen, J.; Huang, S.F.; Guo, W.J.; Bian, X.H.; Wang, F.J.; Li, F.; et al. Ten-Year Survival Outcomes for Patients with Nasopharyngeal Carcinoma Receiving Intensity-Modulated Radiotherapy: An Analysis of 614 Patients from a Single Center. Oral Oncol. 2017, 69, 26–32. [Google Scholar] [CrossRef]
- Au, K.H.; Ngan, R.K.C.; Ng, A.W.Y.; Poon, D.M.C.; Ng, W.T.; Yuen, K.T.; Lee, V.H.F.; Tung, S.Y.; Chan, A.T.C.; Sze, H.C.K.; et al. Treatment Outcomes of Nasopharyngeal Carcinoma in Modern Era after Intensity Modulated Radiotherapy (IMRT) in Hong Kong: A Report of 3328 Patients (HKNPCSG 1301 Study). Oral Oncol. 2018, 77, 16–21. [Google Scholar] [CrossRef]
- Blanchard, P.; Gunn, G.B.; Lin, A.; Foote, R.L.; Lee, N.Y.; Frank, S.J. Proton Therapy for Head and Neck Cancers. Semin. Radiat. Oncol. 2018, 28, 53–63. [Google Scholar] [CrossRef]
- Minatogawa, H.; Yasuda, K.; Dekura, Y.; Takao, S.; Matsuura, T.; Yoshimura, T.; Suzuki, R.; Yokota, I.; Fujima, N.; Onimaru, R.; et al. Potential Benefits of Adaptive Intensity-Modulated Proton Therapy in Nasopharyngeal Carcinomas. J. Appl. Clin. Med. Phys. 2021, 22, 174–183. [Google Scholar] [CrossRef]
- Widesott, L.; Pierelli, A.; Fiorino, C.; Dell’Oca, I.; Broggi, S.; Cattaneo, G.M.; Di Muzio, N.; Fazio, F.; Calandrino, R.; Schwarz, M. Intensity-Modulated Proton Therapy Versus Helical Tomotherapy in Nasopharynx Cancer: Planning Comparison and NTCP Evaluation. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 589–596. [Google Scholar] [CrossRef]
- Lewis, G.D.; Holliday, E.B.; Kocak-Uzel, E.; Hernandez, M.; Garden, A.S.; Rosenthal, D.I.; Frank, S.J. Intensity-Modulated Proton Therapy for Nasopharyngeal Carcinoma: Decreased Radiation Dose to Normal Structures and Encouraging Clinical Outcomes. Head Neck 2016, 38, E1886–E1895. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Urie, M.; Chisin, R.; Suit, H. Proton Therapy for Carcinoma of the Nasopharynx: A Study in Comparative Treatment Planning. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 1607–1614. [Google Scholar] [CrossRef]
- Cheraghi, S.; Nikoofar, P.; Fadavi, P.; Bakhshandeh, M.; Khoie, S.; Gharehbagh, E.J.; Farahani, S.; Mohebbi, A.; Vasheghani, M.; Zare, M.; et al. Short-Term Cohort Study on Sensorineural Hearing Changes in Head and Neck Radiotherapy. Med. Oncol. 2015, 32, 200. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Kadkhoda, Z.; Björk-Eriksson, T.; Nill, S.; Wilkens, J.J.; Oelfke, U.; Johansson, K.A.; Huber, P.E.; Münter, M.W. Intensity-Modulated Radiotherapy of Nasopharyngeal Carcinoma: A Comparative Treatment Planning Study of Photons and Protons. Radiat. Oncol. 2008, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Kitpanit, S.; Lee, A.; Mah, D.; Sine, K.; Sherman, E.; Dunn, L.; Michel, L.; Fetten, J.; Zakeri, K.; et al. Toxicity Profiles and Survival Outcomes Among Patients with Nonmetastatic Nasopharyngeal Carcinoma Treated with Intensity-Modulated Proton Therapy vs Intensity-Modulated Radiation Therapy. JAMA Netw. Open 2021, 4, e2113205. [Google Scholar] [CrossRef]
- Langendijk, J.A.; Boersma, L.J.; Rasch, C.R.N.; van Vulpen, M.; Reitsma, J.B.; van der Schaaf, A.; Schuit, E. Clinical Trial Strategies to Compare Protons with Photons. Semin. Radiat. Oncol. 2018, 28, 79–87. [Google Scholar] [CrossRef]
- Langendijk, J.A.; Lambin, P.; De Ruysscher, D.; Widder, J.; Bos, M.; Verheij, M. Selection of Patients for Radiotherapy with Protons Aiming at Reduction of Side Effects: The Model-Based Approach. Radiother. Oncol. 2013, 107, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.C.; Langendijk, J.A.; Grau, C.; Thariat, J. Proton Therapy and the European Particle Therapy Network: The Past, Present and Future. Cancer/Radiotherapie 2020, 24, 687–690. [Google Scholar] [CrossRef]
- Dijkema, T.; Raaijmakers, C.P.J.; Braam, P.M.; Roesink, J.M.; Monninkhof, E.M.; Terhaard, C.H.J. Xerostomia: A Day and Night Difference. Radiother. Oncol. 2012, 104, 219–223. [Google Scholar] [CrossRef]
- Orlandi, E.; Iacovelli, N.A.; Rancati, T.; Cicchetti, A.; Bossi, P.; Pignoli, E.; Bergamini, C.; Licitra, L.; Carlo, F.; Valdagni, R.; et al. Multivariable Model for Predicting Acute Oral Mucositis during Combined IMRT and Chemotherapy for Locally Advanced Nasopharyngeal Cancer Patients. Oral Oncol. 2018, 86, 266–272. [Google Scholar] [CrossRef]
- Orlandi, E.; Tomatis, S.; Potepan, P.; Bossi, P.; Mongioj, V.; Carrara, M.; Palazzi, M.; Franceschini, M.; Bergamini, C.; Locati, L.; et al. Critical Analysis of Locoregional Failures Following Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma. Future Oncol. 2013, 9, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Chan, A.T.; Licitra, L.; Trama, A.; Orlandi, E.; Hui, E.P.; Halámková, J.; Mattheis, S.; Baujat, B.; Hardillo, J.; et al. Nasopharyngeal Carcinoma: ESMO-EURACAN Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann. Oncol. 2021, 32, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, N.A.; Cicchetti, A.; Cavallo, A.; Alfieri, S.; Locati, L.; Ivaldi, E.; Ingargiola, R.; Romanello, D.A.; Bossi, P.; Cavalieri, S.; et al. Role of IMRT/VMAT-Based Dose and Volume Parameters in Predicting 5-Year Local Control and Survival in Nasopharyngeal Cancer Patients. Front. Oncol. 2020, 10, 518110. [Google Scholar] [CrossRef]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Ten Haken, R.K.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of Normal Tissue Complication Probability Models in the Clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S10–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paganetti, H.; Blakely, E.; Carabe-Fernandez, A.; Carlson, D.J.; Das, I.J.; Dong, L.; Grosshans, D.; Held, K.D.; Mohan, R.; Moiseenko, V.; et al. Report of the AAPM TG-256 on the Relative Biological Effectiveness of Proton Beams in Radiation Therapy. Med. Phys. 2019, 46, e53–e78. [Google Scholar] [CrossRef] [Green Version]
- Ricotti, R.; Pella, A.; Tagaste, B.; Elisei, G.; Fontana, G.; Bonora, M.; Ciocca, M.; Valvo, F.; Orecchia, R.; Baroni, G. Long-Time Clinical Experience in Patient Setup for Several Particle Therapy Clinical Indications: Management of Patient Positioning and Evaluation of Setup Reproducibility and Stability. Br. J. Radiol. 2020, 93, 20190595. [Google Scholar] [CrossRef] [PubMed]
- Stieb, S.; Lee, A.; van Dijk, L.V.; Frank, S.; Fuller, C.D.; Blanchard, P. NTCP Modeling of Late Effects for Head and Neck Cancer: A Systematic Review. Int. J. Part. Ther. 2021, 8, 95. [Google Scholar] [CrossRef]
- Collins, G.; Reitsma, J.; Altman, D.; Moons, K. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Br. J. Surg. 2015, 102, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Landelijk Indicatie Protocol Protonentherapie (Versie 2.2) (LIPPv2.2). Available online: https://docplayer.nl/195792198-Landelijk-indicatie-protocol-protonentherapie-versie-2-2-lippv2-2.html (accessed on 1 April 2021).
- Niyazi, M.; Niemierko, A.; Paganetti, H.; Söhn, M.; Schapira, E.; Goldberg, S.; Adams, J.; Kim, V.; Oh, K.S.; Hwang, W.L.; et al. Volumetric and Actuarial Analysis of Brain Necrosis in Proton Therapy Using a Novel Mixture Cure Model. Radiother. Oncol. 2020, 142, 154–161. [Google Scholar] [CrossRef]
- Loizeau, N.; Fabiano, S.; Papp, D.; Stützer, K.; Jakobi, A.; Bandurska-Luque, A.; Troost, E.G.C.; Richter, C.; Unkelbach, J. Optimal Allocation of Proton Therapy Slots in Combined Proton-Photon Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 196–207. [Google Scholar] [CrossRef]
- Rancati, T.; Fiorino, C. Modelling Radiotherapy Side Effects; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; ISBN 9781138198098. [Google Scholar]
- Lindblom, U.; Gärskog, O.; Kjellén, E.; Laurell, G.; Levring Jäghagen, E.; Wahlberg, P.; Zackrisson, B.; Nilsson, P. Radiation-Induced Trismus in the ARTSCAN Head and Neck Trial. Acta Oncol. 2014, 53, 620–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, C.; Zhu, X.Z.; Lee, T.F.; Feng, P.B.; Xu, J.H.; Qian, P.D.; Zhang, L.F.; He, X.; Huang, S.F.; Zhang, Y.Q. LASSO-Based NTCP Model for Radiation-Induced Temporal Lobe Injury Developing after Intensity-Modulated Radiotherapy of Nasopharyngeal Carcinoma. Sci. Rep. 2016, 6, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Palorini, F.; Cavallo, A.; Ferella, L.; Orlandi, E. Central Nervous System (Brain, Brainstem, Spinal Cord), Ears, Ocular Toxicity. In Modelling Radiotherapy Side Effects: Practical Applications for Planning Optimisation; CRC Press: Boca Raton, FL, USA, 2019; pp. 171–206. [Google Scholar] [CrossRef]
- Rancati, T.; Fiorino, C.; Sanguineti, G. NTCP Modeling of Subacute/Late Laryngeal Edema Scored by Fiberoptic Examination. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Eisbruch, A.; Kim, H.M.; Feng, F.Y.; Lyden, T.H.; Haxer, M.J.; Feng, M.; Worden, F.P.; Bradford, C.R.; Prince, M.E.; Moyer, J.S.; et al. Chemo-IMRT of Oropharyngeal Cancer Aiming to Reduce Dysphagia: Swallowing Organs Late Complication Probabilities and Dosimetric Correlates. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e93–e99. [Google Scholar] [CrossRef] [Green Version]
- Bhide, S.A.; Gulliford, S.; Schick, U.; Miah, A.; Zaidi, S.; Newbold, K.; Nutting, C.M.; Harrington, K.J. Dose-Response Analysis of Acute Oral Mucositis and Pharyngeal Dysphagia in Patients Receiving Induction Chemotherapy Followed by Concomitant Chemo-IMRT for Head and Neck Cancer. Radiother. Oncol. 2012, 103, 88–91. [Google Scholar] [CrossRef]
- Christianen, M.E.M.C.; Schilstra, C.; Beetz, I.; Muijs, C.T.; Chouvalova, O.; Burlage, F.R.; Doornaert, P.; Koken, P.W.; Leemans, C.R.; Rinkel, R.N.P.M.; et al. Predictive Modelling for Swallowing Dysfunction after Primary (Chemo)Radiation: Results of a Prospective Observational Study. Radiother. Oncol. 2012, 105, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Roesink, J.M.; Moerland, M.A.; Battermann, J.J.; Hordijk, G.J.; Terhaard, C.H.J. Qantitative Dose-Volume Response Analysis of Changes in Parotid Gland Function after Radiotheraphy in the Head-and-Neck Region. Int. J. Radiat. Oncol. 2001, 51, 938–946. [Google Scholar] [CrossRef]
- Lee, T.F.; Yeh, S.A.; Chao, P.J.; Chang, L.; Chiu, C.L.; Ting, H.M.; Wang, H.Y.; Huang, Y.J. Normal Tissue Complication Probability Modeling for Cochlea Constraints to Avoid Causing Tinnitus after Head-and-Neck Intensity-Modulated Radiation Therapy. Radiat. Oncol. 2015, 10, 194. [Google Scholar] [CrossRef] [Green Version]
- Vogelius, I.R.; Bentzen, S.M.; Maraldo, M.V.; Petersen, P.M.; Specht, L. Risk Factors for Radiation-Induced Hypothyroidism: A Literature-Based Meta-Analysis. Cancer 2011, 117, 5250–5260. [Google Scholar] [CrossRef]
- Tambas, M.; Steenbakkers, R.J.H.M.; van der Laan, H.P.; Wolters, A.M.; Kierkels, R.G.J.; Scandurra, D.; Korevaar, E.W.; Oldehinkel, E.; van Zon-Meijer, T.W.H.; Both, S.; et al. First Experience with Model-Based Selection of Head and Neck Cancer Patients for Proton Therapy. Radiother. Oncol. 2020, 151, 206–213. [Google Scholar] [CrossRef]
- Ng, S.P.; Pollard, C.; Kamal, M.; Ayoub, Z.; Garden, A.S.; Bahig, H.; Gunn, G.B.; Frank, S.J.; Skinner, H.D.; Phan, J.; et al. Risk of Second Primary Malignancies in Head and Neck Cancer Patients Treated with Definitive Radiotherapy. NPJ Precis. Oncol. 2019, 3, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, J.C.H.; Cheung, K.M.; Au, K.H.; Zee, B.C.Y.; Lee, J.; Ngan, R.K.C.; Lee, A.W.M.; Yiu, H.H.Y.; Li, K.W.S.; Leung, A.K.C.; et al. Radiation-Induced Hypoglossal Nerve Palsy after Definitive Radiotherapy for Nasopharyngeal Carcinoma: Clinical Predictors and Dose-Toxicity Relationship. Radiother. Oncol. 2019, 138, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, L.; van der Schaaf, A.; van der Laan, H.P.; Hoebers, F.J.P.; Wijers, O.B.; van den Hoek, J.G.M.; Moons, K.G.M.; Reitsma, J.B.; Steenbakkers, R.J.H.M.; Schuit, E.; et al. Comprehensive Toxicity Risk Profiling in Radiation Therapy for Head and Neck Cancer: A New Concept for Individually Optimised Treatment. Radiother. Oncol. 2021, 157, 147–154. [Google Scholar] [CrossRef]
- Stuschke, M.; Kaiser, A.; Jawad, J.A.; Pöttgen, C.; Levegrün, S.; Farr, J. Multi-Scenario Based Robust Intensity-Modulated Proton Therapy (IMPT) Plans Can Account for Set-up Errors More Effectively in Terms of Normal Tissue Sparing than Planning Target Volume (PTV) Based Intensity-Modulated Photon Plans in the Head and Neck Region. Radiat. Oncol. 2013, 8, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dijk, L.V.; Steenbakkers, R.J.H.M.; Ten Haken, B.; Van Der Laan, H.P.; Van’t Veld, A.A.; Langendijk, J.A.; Korevaar, E.W. Robust Intensity Modulated Proton Therapy (IMPT) Increases Estimated Clinical Benefit in Head and Neck Cancer Patients. PLoS ONE 2016, 11, e0152477. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.; Wong, A.J.; Gunn, G.B.; Garden, A.S.; Mohamed, A.S.R.; Rosenthal, D.I.; Crutison, J.; Wu, R.; Zhang, X.; Zhu, X.R.; et al. Toward a Model-Based Patient Selection Strategy for Proton Therapy: External Validation of Photon-Derived Normal Tissue Complication Probability Models in a Head and Neck Proton Therapy Cohort. Radiother. Oncol. 2016, 121, 381–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobi, A.; Stützer, K.; Bandurska-Luque, A.; Löck, S.; Haase, R.; Wack, L.-J.; Mönnich, D.; Thorwarth, D.; Perez, D.; Lühr, A.; et al. NTCP Reduction for Advanced Head and Neck Cancer Patients Using Proton Therapy for Complete or Sequential Boost Treatment versus Photon Therapy. Acta Oncol. 2015, 54, 1658–1664. [Google Scholar] [CrossRef] [Green Version]
- Brodin, N.P.; Kabarriti, R.; Pankuch, M.; Schechter, C.B.; Gondi, V.; Kalnicki, S.; Guha, C.; Garg, M.K.; Tomé, W.A. A Quantitative Clinical Decision–Support Strategy Identifying Which Patients with Oropharyngeal Head and Neck Cancer May Benefit the Most from Proton Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 540–552. [Google Scholar] [CrossRef]


| NTCP Model | MBS | MBS Thresholds | CTS Weights | |||
|---|---|---|---|---|---|---|
| Author | Organ | Endpoint (Time post-RT) | Young | Standard | ||
| Niyazi (2020) [30] | Brain | Necrosis > grade II (2 years) | yes | 5% | 10% | 0.1 |
| Kong (2016) [34] | Temporal Lobe | Temporal Lobe Infarction (>3 months) | 0.1 | |||
| Palorini (2019) [35] | Optic Pathways | Radiation Induced Ocular Toxicity (RION) (>3 months) | yes | 3% | 5% | 0.07 |
| Palorini (2019) [35] | Optic Pathways | Grade IV Visual Acuity Loss (>3 months) | yes | 3% | 5% | 0.07 |
| Rancati (2009) [36] | Glottic | Edema grade II (15 months) | 0.005 | |||
| Eisbruch (2011) [37] | Glottic | Aspiration (12 months) | 0.005 | |||
| Bhide (2012) [38] | Oral Cavity | Mucositis (8 weeks) | yes | 10% | 10% | 0.05 |
| Eisbruch (2011) [37] | Superior PCM | Aspiration (12 months) | 0.005 | |||
| Loizeau (2021) [31] | Superior PCM | Grade II-IV dysphagia (6 months) | yes | 3% | 5% | 0.2 |
| Christianen (2012) [39] | Superior PCM | Problems swallowing solids (6 months) | 0.005 | |||
| Christianen (2012) [39] | Superior PCM | Problems swallowing liquids (6 months) | 0.005 | |||
| Loizeau (2021) [31] | Parotid | Moderate to severe xerostomia (6 months) | yes | 15% | 15% | 0.2 |
| Roesink (2001) [40] | Parotid | Flow ratio < 25% (1 year) | 0.005 | |||
| Lee (2015) [41] | Cochlea | Tinnitus (2 years) | 0.01 | |||
| Lindblom (2014) [33] | TMJ | Trismus (>3 months) | 7.5% | 10% | 0.15 | |
| Vogelius (2011) [42] | Thyroid | Hypothyroidism (2 years) | 0.02 | |||
| Total | Percentage (%) | ||
|---|---|---|---|
| All patients | 50 | 100 | |
| Histology | Keratinizing squamous cell carcinoma (WHO type 1) | 3 | 6 |
| Undifferentiated (WHO type 2) | 47 | 94 | |
| Stage T | 1 | 20 | 40.0 |
| 2 | 7 | 14.0 | |
| 3 | 15 | 30.0 | |
| 4 | 8 | 16.0 | |
| Stage N | 0 | 8 | 16.0 |
| 1 | 14 | 28.0 | |
| 2 | 15 | 30.0 | |
| 3 | 13 | 26.0 | |
| Stage | I | 2 | 4.0 |
| II | 7 | 14.0 | |
| III | 19 | 38.0 | |
| IVA | 10 | 20.0 | |
| IVB | 12 | 24.0 | |
| Treatment | RT alone | 4 | 8.0 |
| RT-CHT | 27 | 54.0 | |
| iCHT + RT-CHT | 19 | 38.0 | |
| Target Volume (cc) [mean ± standard deviation] | PTV HD | 239.5 ± 111.2 | |
| PTV ID | 447.1 ± 200.5 | ||
| PTV LD | 612.2 ± 129.9 |
| Classification | All Patients | Tumor Staging | Nodal Involvement | ||||
|---|---|---|---|---|---|---|---|
| Subgroups | Adult | Young | T1T2 | T3T4 | N0 | N1 | N2N3 |
| Number of patients | n = 50 | n = 27 | n = 23 | n = 8 | n = 14 | n = 28 | |
| PT eligibility | |||||||
| Standard | 40.0% | 25.9% | 54.2% | 12.5% | 42.9% | 46.4% | |
| Young | 84.0% | 79.9% | 89.1% | 61.3% | 84.0% | 90.7% | |
| Passing rates | |||||||
| for threshold: | |||||||
| Single | 36.0% | 84.0% | 22.2% | 50.0% | 12.5% | 42.9% | 39.3% |
| Composite | 20.0% | 18.0% | 11.1% | 29.2% | 12.5% | 21.4% | 21.4% |
| for each model: | |||||||
| Brain Necrosis > G2 (2 years) | 8.0% | 46.0% | 3.7% | 12.5% | 0.0% | 14.3% | 7.1% |
| RION/G4 Visual Acuity Loss (>3 months) | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Mucositis (8 weeks) | 6.0% | 6.0% | 0.0% | 12.5% | 12.5% | 7.1% | 3.6% |
| Dysphagia (6 months) | 2.0% | 12.0% | 0.0% | 4.2% | 12.5% | 0.0% | 0.0% |
| Xerostomia (1 year) | 24.0% | 66.0% | 18.5% | 29.2% | 0.0% | 14.3% | 35.7% |
| Trismus (>3 months) | 6.0% | 6.0% | 7.4% | 4.2% | 0.0% | 7.1% | 7.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vai, A.; Molinelli, S.; Rossi, E.; Iacovelli, N.A.; Magro, G.; Cavallo, A.; Pignoli, E.; Rancati, T.; Mirandola, A.; Russo, S.; et al. Proton Radiation Therapy for Nasopharyngeal Cancer Patients: Dosimetric and NTCP Evaluation Supporting Clinical Decision. Cancers 2022, 14, 1109. https://doi.org/10.3390/cancers14051109
Vai A, Molinelli S, Rossi E, Iacovelli NA, Magro G, Cavallo A, Pignoli E, Rancati T, Mirandola A, Russo S, et al. Proton Radiation Therapy for Nasopharyngeal Cancer Patients: Dosimetric and NTCP Evaluation Supporting Clinical Decision. Cancers. 2022; 14(5):1109. https://doi.org/10.3390/cancers14051109
Chicago/Turabian StyleVai, Alessandro, Silvia Molinelli, Eleonora Rossi, Nicola Alessandro Iacovelli, Giuseppe Magro, Anna Cavallo, Emanuele Pignoli, Tiziana Rancati, Alfredo Mirandola, Stefania Russo, and et al. 2022. "Proton Radiation Therapy for Nasopharyngeal Cancer Patients: Dosimetric and NTCP Evaluation Supporting Clinical Decision" Cancers 14, no. 5: 1109. https://doi.org/10.3390/cancers14051109
APA StyleVai, A., Molinelli, S., Rossi, E., Iacovelli, N. A., Magro, G., Cavallo, A., Pignoli, E., Rancati, T., Mirandola, A., Russo, S., Ingargiola, R., Vischioni, B., Bonora, M., Ronchi, S., Ciocca, M., & Orlandi, E. (2022). Proton Radiation Therapy for Nasopharyngeal Cancer Patients: Dosimetric and NTCP Evaluation Supporting Clinical Decision. Cancers, 14(5), 1109. https://doi.org/10.3390/cancers14051109