An Aminosteroid Derivative Shows Higher In Vitro and In Vivo Potencies than Gold Standard Drugs in Androgen-Dependent Prostate Cancer Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. PCa Cell Viability Proliferation (Screening Assays with VCaP, 22Rv1, and LAPC-4)
2.3. LAPC-4 Cell Viability Proliferation and Combination Index Assays
2.4. Apoptosis Characterization by Flow Cytometry
2.5. Gene Expression Study by qPCR Analysis
2.6. Effect of RM-581 in LAPC-4 Xenograft Experiment
2.7. Dosage of RM-581 in Tumors and Plasma
2.8. Tolerated Dose of RM-581 in Mice
2.9. Fatty Acid Determination in LAPC-4 Tumors
2.10. Cholesterol Determination in LAPC-4 Cells, Tumors, Liver, and Plasma
2.11. Cholesterol Determination in Plasma from LAPC-4 Xenograft Experiments
2.12. Histopathology of Mouse Prostate, Kidneys, and Liver from LAPC-4 Xenograft Experiments
2.13. Statistical Analysis
3. Results
3.1. Antiproliferative Activity of RM-581 on Prostate Cancer Cell Lines
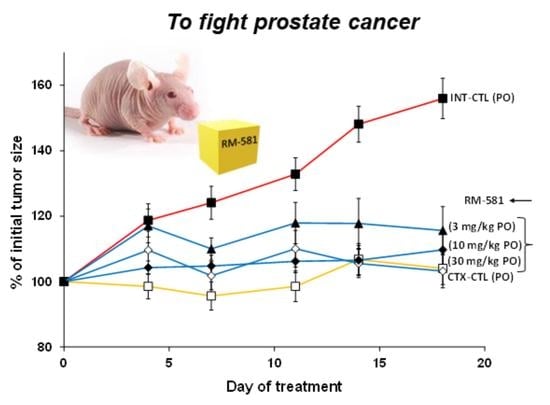
3.2. RM-581 Blocks Tumor Growth in LAPC-4 Xenografts
3.3. Assessment of RM-581 Toxicity
3.4. Cholesterol and Fatty Acid Content Following RM-581 Treatment in LAPC-4 Xenografts
3.5. RM-581 Treatment in LAPC-4 Cells (Genes and Cholesterol)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, M.M.; Hoffman, K.E.; Levy, L.B.; Frank, S.J.; Pugh, T.J.; Choi, S.; Nguyen, Q.N.; McGuire, S.E.; Lee, A.K.; Kuban, D.A. Improvement in prostate cancer survival over time: A 20-year analysis. Cancer. J. 2012, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France. Available online: https://gco.iarc.fr/today (accessed on 12 April 2022).
- Cancer Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 12 April 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer. J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Holm, H.V.; Dahl, A.A.; Klepp, O.H.; Fossa, S.D. Modern treatment of metastatic prostate cancer. Tidsskr. Den Nor. Legeforening 2017, 137, 803–805. [Google Scholar] [CrossRef] [Green Version]
- Roy, J.; DeRoy, P.; Poirier, D. 2beta-(N-substituted piperazino)-5alpha-androstane-3alpha,17beta-diols: Parallel solid-phase synthesis and antiproliferative activity on human leukemia HL-60 cells. J. Comb. Chem. 2007, 9, 347–358. [Google Scholar] [CrossRef]
- Roy, J.; Maltais, R.; Jegham, H.; Poirier, D. Libraries of 2beta-(N-substituted piperazino)-5alpha-androstane-3alpha, 17beta-diols: Chemical synthesis and cytotoxic effects on human leukemia HL-60 cells and on normal lymphocytes. Mol. Divers. 2011, 15, 317–339. [Google Scholar] [CrossRef]
- Maltais, R.; Hospital, A.; Delhomme, A.; Roy, J.; Poirier, D. Chemical synthesis, NMR analysis and evaluation on a cancer xenograft model (HL-60) of the aminosteroid derivative RM-133. Steroids 2014, 82, 68–76. [Google Scholar] [CrossRef]
- Jegham, H.; Maltais, R.; Dufour, P.; Roy, J.; Poirier, D. Solid-phase chemical synthesis and in vitro biological evaluation of novel 2β-piperazino-(20R)-5α-pregnane-3α,20-diol N-derivatives as anti-leukemic agents. Steroids 2012, 77, 1403–1418. [Google Scholar] [CrossRef]
- Jegham, H.; Maltais, R.; Roy, J.; Doillon, C.; Poirier, D. Biological evaluation of a new family of aminosteroids that display a selective toxicity for various malignant cell lines. Anti-Cancer Drugs 2012, 23, 803–814. [Google Scholar] [CrossRef]
- Jegham, H.; Roy, J.; Maltais, R.; Desnoyers, S.; Poirier, D. A novel aminosteroid of the 5α-androstane-3α,17β-diol family induces cell cycle arrest and apoptosis in human promyelocytic leukemia HL-60 cells. Investig. New Drugs 2012, 30, 176–185. [Google Scholar] [CrossRef]
- Ayan, D.; Maltais, R.; Hospital, A.; Poirier, D. Chemical synthesis, cytotoxicity, selectivity and bioavailability of 5α-androstane-3α,17β-diol derivatives. Bioorg. Med. Chem. 2014, 22, 5847–5859. [Google Scholar] [CrossRef]
- Kenmogne, L.C.; Ayan, D.; Roy, J.; Maltais, R.; Poirier, D. The aminosteroid derivative RM-133 shows in vitro and in vivo antitumor activity in human ovarian and pancreatic cancers. PLoS ONE 2015, 10, e0144890. [Google Scholar] [CrossRef] [Green Version]
- Perreault, M.; Maltais, R.; Kenmogne, L.C.; Letourneau, D.; LeHoux, J.G.; Gobeil, S.; Poirier, D. Implication of STARD5 and cholesterol homeostasis disturbance in the endoplasmic reticulum stress-related response induced by pro-apoptotic aminosteroid RM-133. Pharmacol. Res. 2018, 128, 52–60. [Google Scholar] [CrossRef]
- Perreault, M.; Maltais, R.; Roy, J.; Dutour, R.; Poirier, D. Design of a mestranol 2-N-piperazino-substituted derivative showing potent and selective in vitro and in vivo activities in MCF-7 breast cancer models. ChemMedChem 2017, 12, 177–182. [Google Scholar] [CrossRef]
- Dutour, R.; Maltais, R.; Perreault, M.; Roy, J.; Poirier, D. Parallel solid-phase synthesis using a new diethylsilylacetylenic linker and leading to mestranol derivatives with potent antiproliferative activities on multiple cancer cell lines. Anti.-Cancer Agents Med. Chem. 2018, 18, 1469–1481. [Google Scholar] [CrossRef]
- Maltais, R.; Perreault, M.; Roy, J.; Poirier, D. Minor chemical modifications of the aminosteroid derivative RM-581 lead to major impact on its anticancer activity, metabolic stability and aqueous solubility. Eur. J. Med. Chem. 2020, 188, 111990. [Google Scholar] [CrossRef]
- Perreault, M.; Maltais, R.; Roy, J.; Picard, S.; Popa, I.; Bertrand, N.; Poirier, D. Induction of endoplasmic reticulum stress by aminosteroid derivative RM-581 leads to tumor regression in PANC-1 xenograft model. Investig. New Drugs 2019, 37, 431–440. [Google Scholar] [CrossRef]
- Maltais, R.; Roy, J.; Perreault, M.; Sato, S.; Lévesque, J.C.; Poirier, D. Induction of endoplasmic reticulum stress-mediated apoptosis by aminosteroid RM-581 efficiently blocks the growth of PC-3 cancer cells and tumors resistant or not to docetaxel. Int. J. Mol. Sci. 2022, 22, 11181. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the CHOU-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Benítez, F.; Roy, J.; Perreault, M.; Maltais, R.; Poirier, D. A- and D-ring structural modifications of an androsterone derivative inhibiting 17β-hydroxysteroid dehydrogenase type 3: Chemical synthesis and structure-activity relationships. J. Med. Chem. 2019, 62, 7070–7088. [Google Scholar] [CrossRef] [PubMed]
- Moreel, X.; Allaire, J.; Leger, C.; Caron, A.; Labonte, M.E.; Lamarche, B.; Julien, P.; Desmeules, P.; Tetu, B.; Fradet, V. Prostatic and dietary omega-3 fatty acids and prostate cancer progression during active surveillance. Cancer Prev. Res. 2014, 7, 766–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gevariya, N.; Besancon, M.; Robitaille, K.; Picard, V.; Diabate, L.; Alesawi, A.; Julien, P.; Fradet, Y.; Bergeron, A.; Fradet, V. Omega-3 fatty acids decrease prostate cancer progression associated with an anti-tumor immune response in eugonadal and castrated mice. Prostate 2019, 79, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Cholesterol Quantification Kit. Available online: https://www.sigmaaldrich.com/CA/en/product/sigma/mak043 (accessed on 28 May 2023).
- Kramer, C.Y. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics 1956, 12, 307–310. [Google Scholar] [CrossRef]
- Sobel, R.E.; Sadar, M.D. Cell lines used in prostate cancer research: A compendium of old and new lines—Part 1. J. Urol. 2005, 173, 342–359. [Google Scholar] [CrossRef]
- Sobel, R.E.; Sadar, M.D. Cell lines used in prostate cancer research: A compendium of old and new lines—Part 2. J. Urol. 2005, 173, 360–372. [Google Scholar] [CrossRef]
- Jung, M.E.; Ouk, S.; Yoo, D.; Sawyers, C.L.; Chen, C.; Tran, C.; Wongvipat, J. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC). J. Med. Chem. 2010, 53, 2779–2796. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Su, L.; Geng, J.; Liu, J.; Zhao, G. Developments in nonsteroidal antiandrogens targeting the androgen receptor. ChemMedChem 2010, 5, 1651–1661. [Google Scholar] [CrossRef]
- Keating, G.M. Enzalutamide: A review of its use in chemotherapy-naïve metastatic castration-resistant prostate cancer. Drugs Aging 2015, 32, 243–249. [Google Scholar] [CrossRef]
- deVore, N.M.; Scott, E.E. Structure of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature 2012, 482, 116–120. [Google Scholar] [CrossRef] [Green Version]
- de Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B.J.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Berthold, D.R.; Pond, G.R.; Soban, F.; de Wit, R.; Eisenberger, M.; Tannock, I.F. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J. Clin. Oncol. 2008, 26, 242–245. [Google Scholar] [CrossRef]
- Yvon, A.M.C.; Wadsworth, P.; Jordan, M.A. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol. Biol. Cell 1999, 10, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Dang, Q.; Chen, Y.A.; Hsieh, J.T. The dysfunctional lipids in prostate cancer. Am. J. Clin. Exp. Urol. 2019, 7, 273–280. [Google Scholar]
- Maltais, R.; Roy, J.; Poirier, D. Turning a quinoline-based steroidal anticancer agent into fluorescent dye for its tracking by cell imaging. ACS Med. Chem. Lett. 2021, 12, 822–826. [Google Scholar] [CrossRef]
- Sahin, I.H.; Lowery, M.A.; Stadler, Z.K.; Salo-Mullen, E.; Iacobuzio-Donahue, C.A.; Kelsen, D.P.; O’Reilly, E.M. Genomic instability in pancreatic adenocarcinoma: A new step towards precision medicine and novel therapeutic approaches. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 893–905. [Google Scholar] [CrossRef] [Green Version]
- Basseri, S.; Austin, R.C. Endoplasmic reticulum stress and lipid metabolism: Mechanisms and therapeutic potential. Biochem. Res. Int. 2012, 2012, 841362. [Google Scholar] [CrossRef]
- Merseburger, A.S.; Haas, G.P.; von Klot, C.A. An update on enzalutamide in the treatment of prostate cancer. Ther. Adv. Urol. 2015, 7, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Pertusati, F.; Ferla, S.; Bassetto, M.; Brancale, A.; Khandil, S.; Westwell, A.D.; McGuigan, C. A new series of bicalutamide, enzalutamide and enobosarm derivatives carrying pentafluorosulfanyl (SF5) and pentafluoroethyl (C2F5) substituents: Improved antiproliferative agents against prostate cancer. Eur. J. Med. Chem. 2019, 180, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Liu, M.; Liby, T.; Bayani, N.; Bucher, E.; Chiotti, K.; Derrick, D.; Chauchereau, A.; Heiser, L.; Alumkal, J.; et al. Enzalutamide response in a panel of prostate cancer cell lines reveals a role for glucocorticoid receptor in enzalutamide resistant disease. Sci. Rep. 2020, 10, 21750. [Google Scholar] [CrossRef] [PubMed]
- Ritch, C.R.; Cookson, M.S. Advances in the management of castration resistant prostate cancer. BMJ 2016, 355, i4405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N.J.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef] [Green Version]
- Maggoria, M.; Bologna, M.; Cerù, M.P.; Possati, L.; Angelucci, A.; Cimini, A.; Miglietta, A.; Bozzo, F.; Margiotta, C.; Muzio, G.; et al. An overview of the effect of linoleic acid and conjugated-linoleic acids on the growth of several tumor cell lines. Int. J. Cancer 2004, 112, 909–919. [Google Scholar] [CrossRef]
- Carillo, C.; Clavia, M.M.; Alonso-Torre, S.R. Antitumor effect of oleic acid; mechanism of a action. A review. Nutr. Hosp. 2012, 27, 1860–1865. [Google Scholar] [CrossRef] [Green Version]
- Furuya, Y.; Sekine, Y.; Kato, H.; Miyazawa, Y.; Koike, H.; Suzuki, K. Low-density lipoprotein receptors play an important role in the inhibition of prostate cancer cell proliferation by statins. Prostate Int. 2016, 4, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Hughes-Fulford, M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int. J. Cancer 2001, 91, 41–45. [Google Scholar] [CrossRef]
- Kato, H.; Nishitoh, H. Stress responses from the endoplasmic reticulum in cancer. Front. Oncol. 2015, 15, 93. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Huang, H.; Farischon, C.; Li, D.; Du, Z.; Zhang, K.; Zheng, X.; Goodin, S. Combined effects of atorvastatin and aspirin on growth and apoptosis in human prostate cancer cells. Oncol. Rep. 2017, 37, 953–960. [Google Scholar] [CrossRef] [Green Version]







| Entry | PC Cell Lines | AR Status 1 | IC50 (μM) 2 |
|---|---|---|---|
| 1 | DU-145 | N | 4.4 [22] |
| 2 | PC-3 | N | 1.2 [22] |
| 3 | LNCaP | P | 1.2 [22] |
| 4 | VCaP | P | 5.78 |
| 5 | 22Rv1 | P | 1.38 |
| 6 | LAPC-4 | P | 0.56 |
| RM-581 Dose (mg/kg) 1 | RM-581 in Tumor (ng/g—µM) 2 | RM-581 in Plasma (ng/mL—µM) 2 | Concentration Index (Tumor/Plasma) 3 |
|---|---|---|---|
| 3 | 50—0.077 | 15—0.023 | 3.3 |
| 10 | 300—0.464 | 30—0.046 | 10 |
| 30 | 940—1.465 | 171—0.265 | 5.5 |
| Tissue | Method 1 | T-CHOL CTL | T-CHOL RM-581 (10 mg/kg) | F-CHOL CTL | F-CHOL RM-581 (10 mg/kg) |
|---|---|---|---|---|---|
| Tumor (µg/g) | A | 4453 ± 630 | 4543 ± 2476 (NS) | 3610 ± 354 | 3270 ± 236 (NS) |
| Liver (µg/g) | A | 2930 ± 67 | 2440 ± 475 (NS) | 2930 ± 408 | 2410 ± 10 (NS) |
| Plasma (µg/mL) | A | 1700 ± 200 | 1820 ± 500 (NS) | 370 ± 40 | 403 ± 120 (NS) |
| Plasma (µg/mL) | B | 1320 ± 167 | 1182 ± 338 (NS) | -- | -- |
| Tissue | T-FA CTL | T-FA RM-581 (10 mg/kg) | % | S-FA CTL | S-FA RM-581 (10 mg/kg) | % | U-FA CTL | U-FA RM-581 (10 mg/kg) | % |
|---|---|---|---|---|---|---|---|---|---|
| Tumor (mg/g) | 20.0 | 24.5 | +22.5 | 7.24 | 8.15 | +12.6 | 12.5 | 16.0 | +28.0 |
| Liver (mg/g) | 28.8 | 34.5 | +19.8 | 10.6 | 12.5 | +17.9 | 18.1 | 21.9 | +21.0 |
| Plasma (µg/mL) | 3.65 | 3.71 | +1.6 | 1.07 | 1.10 | +2.8 | 2.55 | 2.59 | +1.6 |
| Figure 1 | FA (Double Bond) | % of Total FA | FA CTL (mg/g) | FA RM-581 (mg/g) | % | |
|---|---|---|---|---|---|---|
| Palmitic acid | - | 16:0 | 20.8 | 4.395 | 5.095 | +16 |
| Oleic acid (9c) | w9 | 18:1 | 20.1 | 3.665 | 4.920 | +34 |
| Linoleic acid (9c12c) | w6 | 18:2 | 18.1 | 2.840 | 4.435 | +56 |
| Stearic acid | - | 18:0 | 9.61 | 2.275 | 2.355 | +3.5 |
| Arachidonic (5c8c11c14c) | w6 | 20:4 | 8.77 | 2.195 | 2.150 | −2.1 |
| Palmitoleic acid (9c) | w7 | 16:1 | 4.23 | 0.680 | 1.035 | +52 |
| Vaccenic acid (11c) | w7 | 18:1 | 2.82 | 0.655 | 0.690 | +5.3 |
| Dihomo-γ-linolenic acid (8c11c14c) | w6 | 20:3 | 2.35 | 0.520 | 0.580 | +12 |
| Cervonic acid (4c7c10c13c16c19c) | w3 | 22:6 | 2.18 | 0.530 | 0.530 | 0 |
| Adrenic acid-1 (7c10c13c16c) | w6 | 22:4 | 1.54 | 0.350 | 0.380 | +8.6 |
| Myristic acid | - | 14:0 | 1.12 | 0.220 | 0.275 | +25 |
| Adrenic acid-2 (4c7c10c13c16c) | w6 | 22:5 | 1.07 | 0.295 | 0.260 | −12 |
| Alpha-linolenic acid (9c12c15c) | w3 | 18:3 | 0.94 | 0.140 | 0.230 | +64 |
| Dimethoxyhexadecanoic acid | - | 16:0 | 0.68 | 0.170 | 0.170 | 0 |
| Lignoceric acid | - | 24:0 | 0.65 | 0.130 | 0.160 | +23 |
| Docosapentaenoic acid (7c10c13c16c19c) | w3 | 22:5 | 0.57 | 0.120 | 0.140 | +17 |
| Nervonic acid (15c) | w9 | 24:1 | 0.52 | 0.110 | 0.130 | +18 |
| Gene | Path-Way | RM-581 6 h | RM-581 18 h | RM-581 24 h | RM-581 36 h | Athor 6 h | Athor 18 h | Athor 24 h | Athor 36 h |
|---|---|---|---|---|---|---|---|---|---|
| ACLY | A | 0.8 | 0.4 ** | 0.9 | 0.9 | 1.0 | 1.2 | 1.9 * | 2.2 * |
| HMGCS1 | B | 2.7 ** | 3.3 ** | 2.4 ** | 2.0 ** | 1.3 | 3.5 * | 2.7 * | 2.0 * |
| HMGCR | B | 1.9 ** | 1.2 | 2.6 ** | 2.5 ** | 1.4 * | 1.8 * | 2.6 * | 3.5 * |
| MVK | B | 1.2 | 1.6 | 1.0 | 1.0 | 1.5 | 2.0 * | 2.5 * | 2.5 * |
| MVD | B | 2.7 ** | 1.8 ** | 2.1 ** | 1.6 ** | 1.6 * | 2.6 * | 3.5 * | 4.0 * |
| ACAT1 | C | 0.7 * | 0.9 | 1.1 | 1.2 | 1.0 | 1.0 | 1.0 | 1.1 |
| FASN | D | 1.0 | 0.5 ** | 0.6 * | 0.6 * | 1.2 | 1.3 | 1.7 * | 1.8 * |
| SCD1 | D | 0.7 | 0.8 | 2.5 ** | 3.5 ** | 1.4 | 1.6 * | 2.6 * | 4.5 * |
| LDLR | E | 1.4 | 1.8 ** | 2.6 ** | 2.5 ** | 1.0 | 1.8 * | 1.8 * | 2.5 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poirier, D.; Roy, J.; Maltais, R.; Weidmann, C.; Audet-Walsh, É. An Aminosteroid Derivative Shows Higher In Vitro and In Vivo Potencies than Gold Standard Drugs in Androgen-Dependent Prostate Cancer Models. Cancers 2023, 15, 3033. https://doi.org/10.3390/cancers15113033
Poirier D, Roy J, Maltais R, Weidmann C, Audet-Walsh É. An Aminosteroid Derivative Shows Higher In Vitro and In Vivo Potencies than Gold Standard Drugs in Androgen-Dependent Prostate Cancer Models. Cancers. 2023; 15(11):3033. https://doi.org/10.3390/cancers15113033
Chicago/Turabian StylePoirier, Donald, Jenny Roy, René Maltais, Cindy Weidmann, and Étienne Audet-Walsh. 2023. "An Aminosteroid Derivative Shows Higher In Vitro and In Vivo Potencies than Gold Standard Drugs in Androgen-Dependent Prostate Cancer Models" Cancers 15, no. 11: 3033. https://doi.org/10.3390/cancers15113033
APA StylePoirier, D., Roy, J., Maltais, R., Weidmann, C., & Audet-Walsh, É. (2023). An Aminosteroid Derivative Shows Higher In Vitro and In Vivo Potencies than Gold Standard Drugs in Androgen-Dependent Prostate Cancer Models. Cancers, 15(11), 3033. https://doi.org/10.3390/cancers15113033